
ij����ˮ�к�5.00��10-3mol��L-1�� ���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱�
���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱�
��Ϊ��������ˮ�����õ����Բ���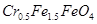 ��
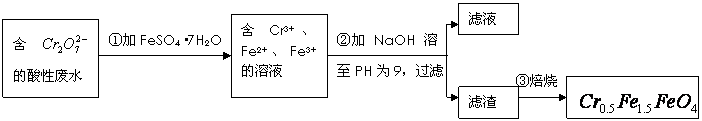
�� �Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�
�Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�


 ��1���ڢٲ���Ӧ�����ӷ���ʽ�� ��
��1���ڢٲ���Ӧ�����ӷ���ʽ�� ��
��2���ڢڲ�����PH��ֽ�ⶨ��ҺPH�IJ��������ǣ�
��
��3���ڢ۲����˵õ�����������Ҫ�ɷֳ�Cr��OH��3�⣬���� ��
��4����ʹ1L�÷�ˮ�е� ��ȫת��Ϊ
��ȫת��Ϊ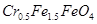 ����������Ҫ����
����������Ҫ����
��FeSO4��7H2O��
��14�֣���1��Cr2O72- + 6Fe2�� + 14H�� ��2Cr3+ + 6Fe3�� + 7H2O��4�֣�
��2����һС��pH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������pH��ֽ�ϣ��������ɫ�����ա���4�֣���3��Fe(OH)3��Fe(OH)2��2�֣���4��13.9��4�֣�
���������������1��Cr2O72-����ǿ�����ԣ��������������ӣ����Ը÷�Ӧ�����ӷ���ʽ��Cr2O72- + 6Fe2�� + 14H�� ��2Cr3+ + 6Fe3�� + 7H2O��
��2����PH��ֽ�ⶨ��ҺPH�IJ��������ǽ�һС��pH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������pH��ֽ�ϣ��������ɫ�����ա�
��3����Һ�к��������Ӻ��������ӣ����Լ�������������Һ��������Cr��OH��3�⣬����Fe(OH)3��Fe(OH)2��
��4��1L�÷�ˮ�е� �����ʵ�����5.00��10-3mol�������ԭ���غ��֪������
�����ʵ�����5.00��10-3mol�������ԭ���غ��֪������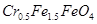 �����ʵ�����2.00��10-2mol�����Ը�����ԭ���غ��֪����ҪFeSO4��7H2O��������2.00��10-2mol��2.5��278g/mol��13.9g��
�����ʵ�����2.00��10-2mol�����Ը�����ԭ���غ��֪����ҪFeSO4��7H2O��������2.00��10-2mol��2.5��278g/mol��13.9g��
���㣺�������ӷ���ʽ����д��pH��ֽ������ҺpHֵ�IJ����Լ��йؼ���
�����������Դ�����ˮ��Cr2O72-Ϊ���壬�ص㿼��ѧ���Ļ�ѧʵ��������������Լ����ݴ����������������ڵ���ѧ����ѧϰ��Ȥ������ѧ����ѧϰ�����ԡ�����Ĺؼ�����ȷʵ��ԭ����Ȼ��������������ü��ɡ������ڽ����йؼ���ʱ��Ҫ���úø����غ㷨���������غ㡢ԭ���غ㡢���ӵĵ�ʧ�غ��Լ�����غ�ȡ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)
![]() ij����ˮ�к�5.00��10-3mol?L-1��
ij����ˮ�к�5.00��10-3mol?L-1��![]() ���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱��Ϊ��������ˮ�����õ����Բ���
���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱��Ϊ��������ˮ�����õ����Բ���![]() ��
��![]() �Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�
�Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�
![]()
![]()
![]()
![]()
��1���ڢٲ���Ӧ�����ӷ���ʽ��
![]() ��2���ڢڲ�����PH��ֽ�ⶨ��ҺPH�IJ����ǣ�
��2���ڢڲ�����PH��ֽ�ⶨ��ҺPH�IJ����ǣ�
![]()
![]() ��3���ڢڲ����˵õ�����������Ҫ�ɷֳ�Cr��OH��3�⣬����
��3���ڢڲ����˵õ�����������Ҫ�ɷֳ�Cr��OH��3�⣬����
![]() ��4����ʹ1L�÷�ˮ�е�
��4����ʹ1L�÷�ˮ�е�![]() ��ȫת��Ϊ
��ȫת��Ϊ![]() ����������Ҫ���� GFeSO4?7H2O��
����������Ҫ���� GFeSO4?7H2O��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����ˮ�к�5.00��10-3mol��L-1��![]() ���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱��Ϊ��������ˮ�����õ����Բ���
���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱��Ϊ��������ˮ�����õ����Բ���![]() ��
��![]() �Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�
�Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�

��1���ڢٲ���Ӧ�����ӷ���ʽ��
��2���ڢڲ�����PH��ֽ�ⶨ��ҺPH�IJ����ǣ�
��3���ڢڲ����˵õ�����������Ҫ�ɷֳ�Cr��OH��3�⣬����
��4����ʹ1L�÷�ˮ�е�![]() ��ȫת��Ϊ
��ȫת��Ϊ![]() ����������Ҫ����
����������Ҫ����
GFeSO4��7H2O��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com