
| ������ | IA | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 1 | ||||||||
| 2 | �� | |||||||
| 3 | �� | �� | �� | �� | �� |

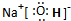
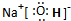
 ��
�� ��
��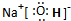 ��
��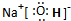 ��
��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Z | ||
| W | X | Y |
| A��Y������������ˮ������ǿ�� |
| B��W������������������ռ���Һ |
| C��Z��������ֻ��һ�� |
| D��X�����������+5�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ʲ�ڶ���ѧ2008��2009ѧ���һ�꼶�ڶ�ѧ�ڵڶ����¿���ѧ���� ���ͣ�022
�±���Ԫ�����ڵ�һ���֣���Ա���Ԫ�أ���д���пհף�
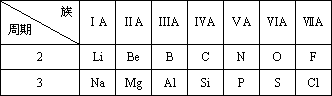
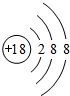
(1)Al3+�Ľṹʾ��ͼΪ________��
(2)NaCl�ĵ���ʽΪ________��
(3)F��Cl��S���⻯�����ȶ�����ǿ����________(���⻯������)
(4)�û�ѧʽ��ʾN��P������������ˮ���������________��________��
(5)Na��Al������������ˮ����֮�䷴Ӧ�Ļ�ѧ����ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±���Ԫ�����ڵ�һ���֣�����������ĸ�ֱ����ijһԪ�ء�
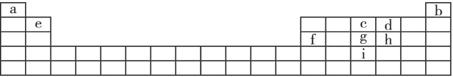
����ݱ�������a��i����Ԫ�أ��ش��������⣺
(1)��̬ԭ�ӵļ۵��Ӳ��У�δ�ɶԵ�������ಢ��ԭ�Ӱ뾶��С��Ԫ�صļ۵��Ӳ�ĵ����Ų�ʽ________________������Ԫ�ص�ԭ����aԪ�ص�ԭ���γɼķ���ʱ����Ԫ�ص�ԭ���ӻ���ʽ______________�����γɼ��ӵ����幹��Ϊ______________���÷���Ϊ______________����(����ԡ��Ǽ��ԡ�)��
(2)d��g��i�ֱ���a�γɻ�����ƶ����γɵĻ�����е��ɸߵ��͵�����˳��Ϊ______________(�ѧʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±���Ԫ�����ڵ�һ���֣�����������ĸ�ֱ����ijһԪ�ء�
| a | b | ||||||||||||||||
| e | c | d | |||||||||||||||
| f | g | h | |||||||||||||||
| j | |||||||||||||||||
����ݱ�������Ԫ�أ��ش��������⣺
��1����̬ԭ�ӵļ۵��Ӳ��У�δ�ɶԵ���������Ԫ���� ����д������ĸ����д����۵��ӵĹ����ʾʽ ������Ԫ�ص�ԭ����aԪ�ص�ԭ���γɼķ���ʱ����Ԫ�ص�ԭ����sp3��ʽ�ӻ������γɼ��ӵ����幹��Ϊ ���÷���Ϊ ���ӣ�����ԡ��Ǽ��ԡ�����
��2��д��jԪ�ػ�̬ԭ�ӵĵ����Ų�ʽ ��d��g��j�ֱ���a�γɻ�����ƶ����γɵĻ�����е��ɸߵ��͵�����˳��Ϊ ���ѧʽ����
��3������������ᡢ�����������������Ҫ��Ԫ���� ����д������ĸ����e��f���ʼ�ef�Ͻ���Ӳ���ɴ�С��˳��Ϊ ����д���ƣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±���Ԫ�����ڵ�һ���֣�����������ĸ�ֱ����ijһԪ�ء�
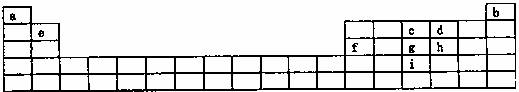
����ݱ�������a i����Ԫ�أ��ش��������⣺
��1����̬ԭ�ӵļ۵��Ӳ��У�δ�ɶԵ�������ಢ��ԭ�Ӱ뾶��С��Ԫ�صļ۵��Ӳ�ĵ����Ų�ʽ ������Ԫ�ص�ԭ����aԪ�ص�ԭ���γɼķ���ʱ����Ԫ�ص�ԭ���ӻ���ʽ �����γɼ��ӵ����幹��Ϊ ���÷���Ϊ ���ӣ�����ԡ��Ǽ��ԡ�����
��2��d��g��I�ֱ���a�γɻ�����ƶ����γɵĻ�����е��ɸߵ��͵�����˳��Ϊ
���ѧʽ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com