
��14�֣�������������������������Ź㷺��Ӧ�ã�ij��ѧ��ȤС������ͼһװ��̽���������й����ʡ�
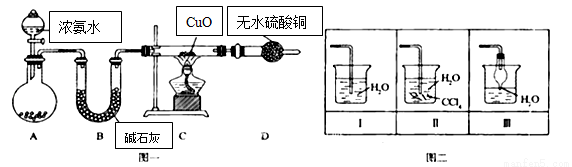
��1��װ��A����ƿ���Լ���ѡ�� ������ţ���B�������� ��
a����ʯ�� b����ʯ�� c��Ũ���� d���ռ���Һ
��2�����Ӻ�װ�ò�����װ�õ������Ժ�װ��ҩƷ��Ȼ��Ӧ�� ����I���
�����������Բ����ƿ�м��백ˮ����װ��C
��3��ʵ���й۲쵽C��CuO��ĩ��죬D����ˮ����ͭ���������ռ���һ�ֵ������壬��÷�Ӧ��ػ�ѧ����ʽΪ ���÷�Ӧ֤���������� �ԣ�
��4����ʵ��ȱ��β������װ�ã�ͼ��������������β����װ���� ����װ����ţ���
��5��������������ˮ������״���£���2��24L�İ�������ˮ���1L��Һ��������Һ�����ʵ���Ũ��Ϊ_______mol/L��
 ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д� һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016�����ʡ����9���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
������������һ������������Na2O2��Ӧ��������й��ɣ��磺Na2O2+SO2=Na2SO4��Na2O2+2SO3=2Na2SO4+O2�����л�ѧ��Ӧ����ʽ�϶�����ȷ��
A��2Na2O2+2Mn2O7=4NaMnO4+O2��
B��2Na2O2+2N2O3=4NaNO2+O2
C��2Na2O2+2N2O5=4NaNO3+O2��
D��Na2O2+2NO2=2NaNO3+O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�����ʡ������ѧ�ڿ�ѧ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����±��ж�Ӧ�������������Dz���������ѡ���ܴﵽ��Ӧʵ��Ŀ�ĵ���
ʵ��Ŀ�� | ʵ�鷽�� | ��ѡ�������� | |
A | ��֤�Ҵ�������ȥ��Ӧ������ϩ | ���Ҵ���Ũ�����ϼ��ȵ�170�棬������������ͨ����ˮ | �ƾ��ơ�Բ����ƿ���������ܡ��Թ� |
B | ����1 L 1.6%��CuSO4��Һ����Һ�ܶȽ���Ϊ1g/mL�� | ��25g CuSO4��5H2O�ܽ���975g ˮ�� | �ձ�����Ͳ�������� |
C | ��֤����ˮ�������л�ԭ�� | ��������Һ�м��뼸��ϡ���ᣬˮԡ���ȼ����ӣ��������м������Ƶ�������Һ����ˮԡ���� | �Թܡ��ձ����ƾ��ơ��ι� |
D | ��֤HClO��CH3COOH������ǿ�� | ��ͬ��������pH��ֽ��0.1mol��L��1 NaClO��Һ��0��1mol��L��1CH3COONa��Һ��pH | ������������Ƭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��㶫ʡ�麣�и���9�������Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
������ɫˮ��Һ�д��������һ��������
A��H+��Fe3+��I-��Cl- B��Al3+��Mg2+��NO3-��Cl-
C��K+��Ag+ ��Ca2+��SO42- D��NH4+��Na+��AlO2-��MnO4-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��㶫ʡ��������У��������ѧ�ڵ�һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ������Ԥ����ȷ����

A��ʵ�����ֹ���ϲ���Һ��ɫ���ֲ���
B��ʵ�������KMnO4��Һ�г������ݣ�����ɫ�ޱ仯
C��ʵ�����ϡHNO3Ƭ�̣���Һ�������ݲ��������ƿ��ʼ�ձ�����ɫ
D��ʵ����������Һ�����ɫ��ֹͣ���ȣ�����ͨ����ϵʱ���������ЧӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�찲��ʡ�쳤�и�����һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���и���������ָ����Һ��һ���ܴ����������
A����ʹ���ȳʺ�ɫ����Һ��Ba2+��Al3+��NO3����Cl-
B����ˮ�������c��H+��=1��10-11 mol��L-1����Һ��Na+��Mg2+��Cl-��NO3��
C��0��2 mol��L-1��NaNO3��Һ��H+��Fe2+��SO42����Cl-
D����Fe��Ӧ����H2����Һ��NH4+��K+��SO42����CO32��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ���Ĵ�ʡ�Ű��и�һ9���¿���ѧ�Ծ��������棩 ���ͣ������
Ϊ�˳�ȥ�Ȼ�����Ʒ�е�����̼���ƣ�ij��ȤС����������������ʾ��������ʵ�顣
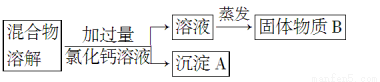
��1������A�Ļ�ѧʽ��________��
��2����������Ȼ�����Һ�����ȥ����A��ʵ�����������________________��
��3��������ʵ������У��ַ������µ����⣺�˷��������������µ����ʡ���������B�ijɷ�Ϊ_____________(�û�ѧʽ��ʾ)��
��4�����Ǽ���̽������������µķ�������������ܽ⣬�μ����������ٲ�������Ϊֹ��Ȼ�������С��йط�Ӧ�Ļ�ѧ����ʽΪ________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ɽ��ʡ��һ��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
(8��)ijͬѧ������ͼ��ʾ��װ�ý����й�ʵ�顣��������A����Ҫ�ɷ�������������������������ˮ��������ش��������⣺
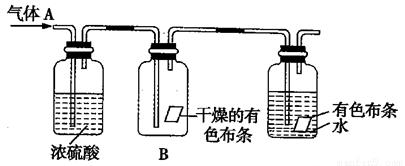
��1����ʵ�����ҪĿ�����о�����������Ƿ���� ���á�
��2��Ũ�����������
��3��ʵ������й۲쵽Bƿ�и������ɫ���� (�A���ˡ���B�����ˡ�)ɫ��
��4���ڸ�ʵ���У�װ�ô���ȱ�ݣ���Ӧ��ʢ�� ��װ�á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��㶫ʡ�����и�һ��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����������Һ�������ͺ��Ȼ�����Һ ��39�����Ҵ���Һ ���Ȼ��ƺ͵������ˮ��Һ���������ϸ����Һ����ȷ����������
A ����Һ����ȡ������ B����ȡ������Һ
C ����Һ��������ȡ D��������ȡ����Һ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com