
| |||||||||||||||||||||||||||||||||||||||
(1) |
ѡ������ӦԽ��ȷԽ�ã���ˮֻռ3����ֻ��0.03 L����30 mL����ѡ��50 mL��Ͳ�� |
(2) |
�����𰸣��ֲ�������(��������̫����)���غӷ�����ȾˮԴ�������ʡ��Ƿ���ھ���ʹ�á���С�����۵�Ӱ�졢�Ծ����ճ������Ӱ�졢�Ƿ���Ⱦ��������������� ����˼·�������Ժ�ˮ��Ⱦ���ص���ȾԴ֮һ��������ˮ����������������С�ӣ��ض����������Ⱦ�� |
(3) |
����������һ�����ӵ����⣬���������������Ⱦ��Ӧ��ʱ��أ���ʱ������������һ�ֳ��õķ�����������Է����ϸ����� |
(4) |
�����������ռ��㣻�����ܣ���������(��ש���Ʒ��ϡ�����������) |
|
���������Ŀ���ǿ��黷����ˮ�ʵ���Ⱦ��Ҫ��ѧ������һ���ķ�������ͽ��������������ܸ���ˮ��Ⱦ�ĸ�����ȾԴ������ѧ֪ʶȥ������ߵ����⣬���ֻ�ѧѧ�Ƶ�ʵ����ԭ�� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
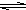 HS-+OH-
HS-+OH-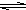 HS-+OH-
HS-+OH-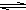 Al��OH��3+3H+����ɫ����Ͱ�ɫ��״��������
Al��OH��3+3H+����ɫ����Ͱ�ɫ��״��������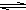 Al��OH��3+3H+����ɫ����Ͱ�ɫ��״��������
Al��OH��3+3H+����ɫ����Ͱ�ɫ��״���������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com