
ij�л���A,��C��H��O����Ԫ����ɣ���һ�������£���A����ת��Ϊ�л���B��C��D��E��C�ֿ���ת��ΪB��A�����ǵ�ת����ϵ���£�

��֪D�������ܶ���������22���������Է���������Ӧ��
��1��д���������ʵĽṹ��ʽ
E ��F ��
��2��д�����в���Ļ�ѧ��Ӧ����ʽ
�� ��
�� ��
�� ��
�� ��
�� ����������Ӧ��
��3��д����F����ͬ���������ܷ���������Ӧ��ͬ���칹��Ľṹ��ʽ
��
 ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ͼ��ʾװ�õ�������������ǣ�(����)
A��п�Ǹ�������������С
B����������ͭƬ���汻��ԭ����������
C��������пƬ����������ͭƬ
D�����Ӵ�пƬ����������ͭƬ

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ���ʵ�ת����ϵ�У�A��һ�ֹ��嵥�ʣ�E��һ�ְ�ɫ������a��Һ��NaOH��Һ��F����ѧ�������ܶ���С�����塣

��ش��������⣺
��1��B�Ļ�ѧʽ�� ��C�Ļ�ѧʽ�� ��
��2��B��a��Һ��Ӧ����������ʽ�� ��
��3��A��a��Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��4��B�����������Ƶ�A��д����ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����뱽�״���ͬ���е�������(����)
A������NaOH��Һ��Ӧ B�������Ʒ�Ӧ�ų�H2
C������������ D����FeCl3��Һ��Ӧ����ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ����ʽΪC8H14N2O5�Ķ��ģ���ˮ��õ������� ����һ�ְ�����X����X�Ļ�ѧʽ������(����)
����һ�ְ�����X����X�Ļ�ѧʽ������(����)
A��C3H7NO3 B��C5H9NO4 C��C5H11NO5 D��C5H7NO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����г��õ�ij������X�Ľṹ��ʽΪ

(1)����X�к��������ŵ�������_________________________________��
(2)����X�ɷ����ķ�Ӧ������____________________________(�����)��
a��������Ӧ������������ b����ԭ��Ӧ
c���ӳɷ�Ӧ d����ȥ��Ӧ
(3)��֪��

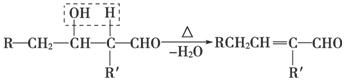
����X�ĺϳ�·�����£�

��A�Ľṹ��ʽ��______________________________________________��
�ڼ����л���C�к���̼̼˫�������õ��Լ�__________________��
a��������Һ������������ b�����Ը��������Һ
c����ˮ d������������Һ
��D��X�Ļ�ѧ����ʽΪ________________________________________��
���л���B��ij��ͬ���칹��E�������������ʣ�
a����Ũ��ˮ��Ӧ���ɰ�ɫ��������1 mol E�������4 mol Br2��Ӧ
b�����������ʾ���л����д���̼̼˫��
��E�Ľṹ��ʽΪ_____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ԫ�ص�ԭ�ӽṹ���������ʺ������ڱ��е�λ�á�����˵����ȷ����
(����)
A������Ԫ��ԭ�ӵ�����������������Ԫ�ص�����ϼ�
B��P��S��Cl�õ�������������������Ӧ��ˮ��������Ծ�������ǿ
C�������ԭ���У�����˽Ͻ����������˶��ĵ��������ϸ�
D��Ԫ�����ڱ���λ�ڽ����ͷǽ����ֽ��߸�����Ԫ�����ڹ���Ԫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л�����ӽṹ�л���֮����Ӱ��ᵼ�����ʻ�ѧ���ʵĸı䣬���и������ʵ����˵�������۵���� ( )
A���ױ���ʹ���Ը��������Һ��ɫ�������鲻��ʹ���Ը��������Һ��ɫ
B���Ҵ������H+����������H2O
C����ϩ�ܷ����ӳɷ�Ӧ�������鲻�ܷ����ӳɷ�Ӧ
D����������NaOH��Һ��Ӧ�����Ҵ�������NaOH��Һ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ԭ�ӵ���Χ�����Ų�ʽ��ȷ����
�� A��S��3p4������B��Cr��3d44s2��������C��Se��5s25p6������D��Cu��3d104s1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com