
2013���,�������������Ű�ҹ��ж�������������,����β����ȼúβ������ɿ�����Ⱦ��ԭ��֮һ��
(1)����β����������Ҫԭ��Ϊ:2NO(g)+2CO(g)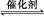 2CO2(g)+N2(g)�����ܱ������з����÷�Ӧʱ,c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯����,��ͼ��ʾ��
2CO2(g)+N2(g)�����ܱ������з����÷�Ӧʱ,c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯����,��ͼ��ʾ��
�ݴ��ж�:
�ٸ÷�Ӧ�Ħ�H����0(�>����<��)��
����T2�¶���,0��2 s�ڵ�ƽ����Ӧ����v(N2)=����������
�۵��������������һ��ʱ,���������������ѧ��Ӧ���ʡ��������ı����S1>S2,����ͼ�л���c(CO2)��T1��S2�����´ﵽƽ������еı仯���ߡ�
�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н���,����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬������������(�����)�� 
(2)ֱ���ŷ�úȼ�ղ������������������صĻ������⡣
��úȼ�ղ���������������������,��CH4����ԭNOx�������������������Ⱦ��
����:
CH4(g)+2NO2(g) N2(g)+CO2(g)+2H2O(g) ��H1="-867" kJ/mol
N2(g)+CO2(g)+2H2O(g) ��H1="-867" kJ/mol
2NO2(g) N2O4(g) ��H2="-56.9" kJ/mol
N2O4(g) ��H2="-56.9" kJ/mol
д��CH4(g)����ԭN2O4(g)����N2(g)��H2O(g)���Ȼ�ѧ����ʽ:�� ��
�ڽ�ȼú�����Ķ�����̼��������,�ɴﵽ��̼�ŷŵ�Ŀ�ġ���ͼ��ͨ���˹��������,��CO2��H2OΪԭ���Ʊ�HCOOH��O2��ԭ��ʾ��ͼ������b���淢���ĵ缫��ӦʽΪ�� �� 
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�״�ȼ�Ϸ�Ϊ�״����ͺͼ״����͡���ҵ�Ϻϳɼ״��ķ����ܶࡣ
��1��һ�������·�����Ӧ��
CO2(g) +3H2(g) ��CH3OH(g)+H2O(g) ��H1
2CO (g) +O2(g) ��2CO2(g) ��H2
2H2(g)+O2(g) ��2H2O(g) ��H3
��CO(g) + 2H2(g)  CH3OH(g)���ġ�H�� ��
CH3OH(g)���ġ�H�� ��
��2�����ݻ�Ϊ2L���ܱ������н��з�Ӧ��CO(g)+2H2(g) CH3OH(g) �������������䣬��300���500��ʱ�����ʵ���n(CH3OH) �뷴Ӧʱ��t�ı仯������ͼ��ʾ���÷�Ӧ�ġ�H 0 ����>��<��=����
CH3OH(g) �������������䣬��300���500��ʱ�����ʵ���n(CH3OH) �뷴Ӧʱ��t�ı仯������ͼ��ʾ���÷�Ӧ�ġ�H 0 ����>��<��=����
��3����Ҫ��״��IJ��ʣ��ɲ�ȡ�Ĵ�ʩ��____________������ĸ����
| A����������� |
| B�������¶� |
| C�������¶� |
| D��ʹ�ú��ʵĴ��� |
 CO+3H2��T��ʱ����1 L�ܱ�������Ͷ��1 mol CH4��1 mol H2O(g)��5Сʱ���÷�Ӧ��ϵ�ﵽƽ��״̬����ʱCH4��ת����Ϊ50% ��������¶��µ�ƽ�ⳣ�� ���������С�������λ���֣���
CO+3H2��T��ʱ����1 L�ܱ�������Ͷ��1 mol CH4��1 mol H2O(g)��5Сʱ���÷�Ӧ��ϵ�ﵽƽ��״̬����ʱCH4��ת����Ϊ50% ��������¶��µ�ƽ�ⳣ�� ���������С�������λ���֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�о�CO2�����öԴٽ���̼���Ĺ���������Ҫ���塣
��1����CO2�뽹̿��������CO��CO�����������ȡ�
��֪��Fe2O3(s) + 3C(ʯī) =" 2Fe(s)" + 3CO(g) ��H 1 =" +489.0" kJ��mol��1
C(ʯī) +CO2(g) = 2CO(g) ��H 2 =" +172.5" kJ��mol��1
��CO��ԭFe2O3(s)���Ȼ�ѧ����ʽΪ ��
��2��������̼�ϳɼ״���̼���ŵ��·���CO2ת��Ϊ�״����Ȼ�ѧ����ʽ
CO2(g) +3H2(g) CH3OH(g) +H2O(g) ��H
CH3OH(g) +H2O(g) ��H
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪK= ��
��ȡһ�����CO2��H2�Ļ������(���ʵ���֮��Ϊ1��3)����������ܱ������У�����������Ӧ����Ӧ�����в�ü״������������(CH3OH)�뷴Ӧ�¶�T�Ĺ�ϵ��ͼA��ʾ����÷�Ӧ�Ħ�H 0���>������<����������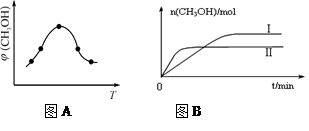
�������ֲ�ͬ�����·�����Ӧ�����CH3OH�����ʵ�����ʱ��仯��ͼB��ʾ������I�����Ӧ��ƽ�ⳣ����С��ϵΪK�� K�����>�� ��<������
��3����CO2Ϊԭ�ϻ����Ժϳɶ������ʡ��ٹ�ҵ������[CO(NH2)2]��CO2��NH3��һ�������ºϳɣ��䷴Ӧ����ʽΪ ������̼��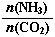 ��3����ƽ��ʱCO2��ת����Ϊ60%����NH3��ƽ��ת����Ϊ ��
��3����ƽ��ʱCO2��ת����Ϊ60%����NH3��ƽ��ת����Ϊ ��
����������Һ������ʽ��е�⣬CO2�ڵ缫�Ͽ�ת��Ϊ���飬�õ缫��Ӧ�ķ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1������ˮú�������������·�Ӧ����C(s)��CO2(g)??2CO(g)����H1��
��CO(g)��H2O(g)  H2(g)��CO2(g)����H2��
H2(g)��CO2(g)����H2��
��C(s)��H2O(g)  CO(g)��H2(g)����H3��
CO(g)��H2(g)����H3��
������Ӧ��H3�릤H1����H2֮��Ĺ�ϵΪ__________________________________��
��2����CH4ת����CO����ҵ�ϳ����ô�ת���������䷴Ӧԭ��Ϊ
2CH4(g)��3O2(g)  2CO(g)��4H2O(g)
2CO(g)��4H2O(g)
��H����1 038 kJ��mol��1��
��ҵ��Ҫѡ����ʵĴ������ֱ��X��Y��Z���ִ�����������ʵ��(����������ͬ)��
��X��750 ��ʱ��Ч����ߣ���ʹ����Ӧ���ʼӿ�Լ3��105����
��Y��600 ��ʱ��Ч����ߣ���ʹ����Ӧ���ʼӿ�Լ3��105����
��Z��440 ��ʱ��Ч����ߣ���ʹ�淴Ӧ���ʼӿ�Լ1��106����
����������Ϣ������Ϊ��������Ӧ��ѡ������˴�����________(�X����Y����Z��)��ѡ���������________________________________________________________��
��3���뻭����2���з�Ӧ���д�����������������·�Ӧ��������ϵ�����仯ʾ��ͼ�������б�Ҫ��ע��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�о�SO2��CO�ȴ�����Ⱦ��Ĵ��������þ����ش����塣
��.�����Ƽ�ѭ�������ѳ�������SO2���÷���Na2SO3��Һ��Ϊ���ռ������չ���pH��n��SO��?n��HSO3-���仯��ϵ���±���
| n��SO32-��?n��HSO3-�� | 91:9 | 1:1 | 9:91 |
| pH | 8.2 | 7.2 | 6.2 |
 CH3OH��g����
CH3OH��g����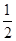 O2��g��=H2O��g������H����241.8 kJ/mol��
O2��g��=H2O��g������H����241.8 kJ/mol��| ��ѧ�� | H��H | H��O | C��H | C��O | C=O |
| ���� | 435 | 463 | 413 | 356 | 745 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����̼ѭ������������ĸ߶����ӣ�����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2��������ȫ������ձ����ӡ����ԡ���̼���á�����Ϊ��ѧ���о�����Ҫ���⡣
��1��д��CO2��H2��Ӧ����CH4��H2O���Ȼ�ѧ����ʽ ��
��֪�� �� CO(g)+H2O(g) H2(g)+CO2(g) ��H����41kJ��mol��1
H2(g)+CO2(g) ��H����41kJ��mol��1
�� C(s)+2H2(g) CH4(g) ��H����73kJ��mol��1
CH4(g) ��H����73kJ��mol��1
�� 2CO(g) C(s)+CO2(g) ��H����171kJ��mol��1
C(s)+CO2(g) ��H����171kJ��mol��1
��2����ȼú�����е�CO2ת��Ϊ�����ѵķ�Ӧԭ��Ϊ��2CO2(g) + 6H2(g) CH3OCH3(g) + 3H2O(g)����֪һ�������£��÷�Ӧ��CO2��ƽ��ת�������¶ȡ�Ͷ�ϱ�[n(H2) / n(CO2)]�ı仯����������ͼ��
CH3OCH3(g) + 3H2O(g)����֪һ�������£��÷�Ӧ��CO2��ƽ��ת�������¶ȡ�Ͷ�ϱ�[n(H2) / n(CO2)]�ı仯����������ͼ��
����������������ʱ��������ͼ�л���ƽ��ʱCH3OCH3�����������Ͷ�ϱ�[n(H2) / n(CO2)]�仯������ͼ��
��ij�¶��£���2.0molCO2(g)��6.0molH2(g)�����ݻ�Ϊ2L���ܱ������У���Ӧ����ƽ��ʱ���ı�ѹǿ���¶ȣ�ƽ����ϵ��CH3OCH3(g)�����ʵ��������仯�����ͼ��ʾ�������¶Ⱥ�ѹǿ�Ĺ�ϵ�ж���ȷ���� ��
A. P3��P2��T3��T2 B. P1��P3��T1��T3 C. P2��P4��T4��T2 D. P1��P4��T2��T3
���ں����ܱ������ﰴ�����Ϊ1:3���������̼���� ����һ�������·�Ӧ�ﵽƽ��״̬�����ı䷴Ӧ��ijһ�����������б仯��˵��ƽ��һ�����淴Ӧ�����ƶ����� ��
A. ����Ӧ������������С
B. �淴Ӧ������������С
C. ��ѧƽ�ⳣ��Kֵ����
D. ��Ӧ�������ٷֺ�������
E. ���������ܶȼ�С
F. ������ת���ʼ�С
��3�������ѧ���ٴ��������ɫ��ѧ�����룺�ѿ�������̼�����Һ��Ȼ���ٰ�CO2����Һ����ȡ����������ѧ��Ӧ��ʹ�����е�CO2ת��Ϊ������ȼ�ϼ״����״�������ȼ�ϵ�أ�д����ϡ����Ϊ����ʼ״�ȼ�ϵ�ظ�����Ӧʽ__ ���Դ�ȼ�ϵ����Ϊ��ӵ�Դ��ͼ��ʾ�������ͭ��Һ�������ʼʱʢ��1000mL pH��5������ͭ��Һ��25�棬CuSO4��������һ��ʱ�����Һ��pH��Ϊ1����ʱ�ɹ۲쵽�������� ����Ҫʹ��Һ�ָ�����ʼŨ�ȣ��¶Ȳ��䣬������Һ����ı仯����������Һ�м��� �����������ƣ���������ԼΪ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪��������Һ�е�ת����ϵ��ͼ��ʾ��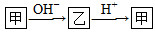 ����ش��������⣺
����ش��������⣺
(1)������10���ӵ������ӣ����Ǽ������塣1 mol��ͨ������ǿ����Һ����H����Ӧ����Ӧ�����е������仯��ͼ��д���ҵ�һ����;________________���÷�Ӧ���Ȼ�ѧ����ʽΪ___________________________��
(2)������CO2����CO2��NH3��Ӧ���Ժϳ����أ��ϳ����صķ�Ӧ��Ϊ����������
��һ����2NH3(l)��CO2(g) H2NCOONH4(l)(���������)����H1
H2NCOONH4(l)(���������)����H1
�ڶ�����H2NCOONH4(l) H2O(l)��H2NCONH2(l)(����)����H2
H2O(l)��H2NCONH2(l)(����)����H2
��һ���Ϊ0.5 L���ܱ�������Ͷ��4 mol����1 mol������̼��ʵ���÷�Ӧ�и���ֵ����ʵ�����ʱ��ı仯��ͼ����ʾ��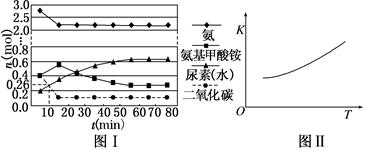
����֪�ܷ�Ӧ�Ŀ���������һ����������ϳ������ܷ�Ӧ�Ŀ����ɵ�________����Ӧ������
�ڷ�Ӧ���е�10 minʱ���CO2�����ʵ�����ͼ����ʾ����ǰ10 min��CO2��ʾ�ĵ�һ����Ӧ������Ϊ________��
�۵ڶ�����Ӧ��ƽ�ⳣ��K���¶ȵı仯��ͼ����ʾ����H2________0(�����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)��֪��
��Fe(s)��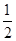 O2(g)=FeO(s)����H����272.0 kJ��mol��1
O2(g)=FeO(s)����H����272.0 kJ��mol��1
��2Al(s)�� O2(g)=Al2O3(s)����H����1675.7 kJ��mol��1
O2(g)=Al2O3(s)����H����1675.7 kJ��mol��1
Al��FeO�������ȷ�Ӧ���Ȼ�ѧ����ʽ��____________________________________
(2)ij���淴Ӧ�ڲ�ͬ�����µķ�Ӧ���̷ֱ�ΪA��B(����ͼ��ʾ)��
�ٸ���ͼ�жϸ÷�Ӧ�ﵽƽ��������������䣬�����¶ȣ���Ӧ���ת����________(�������С�����䡱)��
������B���̱����˷�Ӧ���õ�����Ϊ________(ѡ�����)��
A�������¶ȡ������� B������Ӧ���Ũ�� C�������¶� D��ʹ�ô���
(3)1000 ��ʱ���������������������з�Ӧ��Na2SO4(s)��4H2(g) Na2S(s)��4H2O(g)
Na2S(s)��4H2O(g)
�÷�Ӧ��ƽ�ⳣ������ʽΪ________________________________��
��֪K1000 ��<K1200 ������������ϵ�¶ȣ���������ƽ����Է�����������________(�������С�����䡱)��
(4)�����£����ȡ0.1 mol��L��1 HA��Һ��0.1 mol��L��1 NaOH��Һ��������(��Ϻ���Һ����ı仯���Բ���)����û��Һ��pH��8��
�ٻ��Һ����ˮ�������OH��Ũ����0.1 mol��L��1 NaOH��Һ����ˮ�������OH��Ũ��֮��Ϊ________��
����֪NH4A��ҺΪ���ԣ���֪��HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶ�(NH4)2CO3��Һ��pH________7(�<����>������)����ͬ�¶��£������ʵ���Ũ�ȵ�������������Һ��pH�ɴ�С������˳��Ϊ(�����)________��
a��NH4HCO3 b��NH4A c��(NH4)2CO3 d��NH4Cl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊ�������Դ�����ʣ����ٻ�����Ⱦ���������Ž��ѳ����ȼ�ͼ״�����ɲ�ҵ������ͼ��ʾ��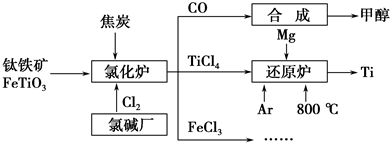
����д���пհף�
(1)����������Ȼ�¯ǰͨ����ȡϴ�ӡ����顢��ɡ�Ԥ�ȵ������������������ԭ���Ͻ��ͷ�������ã�_______________________________________��
��֪�Ȼ�¯�з�Ӧ�����ͽ�̿�������������ʵ�����Ϊ7��6�����Ȼ�¯�еĻ�ԭ����ѧʽ��_________________________________________________��
(2)��֪����Mg(s)��Cl2(g)=MgCl2(s) ��H����641 kJ��mol��1
��2Mg(s)��TiCl4(s)=2MgCl2(s)��Ti(s) ��H����512 kJ��mol��1
��Ti(s)��2Cl2(g)=TiCl4(s)����H��________��
(3)Ar��ͨ�뻹ԭ¯�в������뷴Ӧ��ͨ��Ar����������_________________________________________________________________��
(4)�Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء���֪��ȼ�ϵ�ص��ܷ�ӦʽΪ2CH3OH��3O2��4OH��===2CO32����6H2O���õ���������ϵĵ缫��ӦʽΪ____________________________________________��
����һ��ʱ������Һ��pH________(���С�����������䡱)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com