
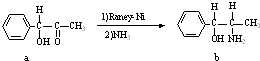
| 1 |
| 8 |
| 1 |
| 2 |
| 1 |
| 8 |
 ��
�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��20��)
19-I(6��)������������ȷ����
A��CS2ΪV�εļ��Է���
B��Cl0�� 3 �Ŀռ乹��Ϊƽ��������
C��SF6����6����ȫ��ͬ�ijɼ����Ӷ�
D��SiF4��SO2�� 3 ������ԭ�Ӿ�Ϊsp3�ӻ�
19-��(14��)���������仯�����ںϽ�����Լ������ȷ���Ӧ�ù㷺����ش��������⣺
(1)Niԭ�ӵĺ�������Ų�ʽΪ______________________________��
(2)Ni0��Fe0�ľ���ṹ���;����Ȼ��Ƶ���ͬ��Ni2+��Fe2+�����Ӱ뾶�ֱ�Ϊ69 pm��78 pm�����۵�NiO ________ FeO(�<����>��)��
(3)Ni0������Ni��O����λ���ֱ�Ϊ_______________��_______________��
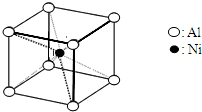
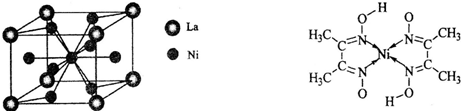
(4)����������(La)�γɵĺϽ���һ�����õĴ�����ϣ��侧���ṹʾ��ͼ������ͼ��ʾ���úϽ�Ļ�ѧʽΪ_______________��
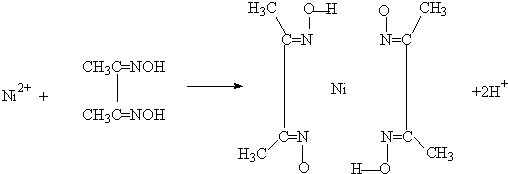
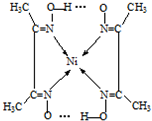
(5)����ͪ뿳����ڼ���Ni2+����ϡ��ˮ�����У�����ͪ���Ni2+��Ӧ�������ʺ�ɫ��������ṹ������ͼ��ʾ��
�ٸýṹ�У�̼̼֮��Ĺ��ۼ�������![]() ����̼��֮��Ĺ��ۼ�������______________������֮���γɵĻ�ѧ����_______________��
����̼��֮��Ĺ��ۼ�������______________������֮���γɵĻ�ѧ����_______________��
�ڸýṹ�У�����֮������ۼ���ɴ���_______________��
�۸ýṹ�У�̼ԭ�ӵ��ӻ����������_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��߿���ѧ�������ר���� ���ʽṹ��Ԫ�������� ���ͣ������
��20��)
19-I(6��)������������ȷ����
| A��CS2ΪV�εļ��Է��� |
| B��Cl0�� 3 �Ŀռ乹��Ϊƽ�������� |
| C��SF6����6����ȫ��ͬ�ijɼ����Ӷ� |
| D��SiF4��SO2�� 3 ������ԭ�Ӿ�Ϊsp3�ӻ� |


 ����̼��֮��Ĺ��ۼ�������______________������֮���γɵĻ�ѧ����_______________��
����̼��֮��Ĺ��ۼ�������______________������֮���γɵĻ�ѧ����_______________�� �۸ýṹ�У�̼ԭ�ӵ��ӻ����������_______________��
�۸ýṹ�У�̼ԭ�ӵ��ӻ����������_______________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010����ͨ�ߵ�ѧУ����ȫ��ͳһ���Ի�ѧ���⣨���Ͼ��� ���ͣ������
��20��)
19-I(6��)������������ȷ����
A��CS2ΪV�εļ��Է���
B��Cl0�� 3 �Ŀռ乹��Ϊƽ��������
C��SF6����6����ȫ��ͬ�ijɼ����Ӷ�
D��SiF4��SO2�� 3 ������ԭ�Ӿ�Ϊsp3�ӻ�
19-��(14��)���������仯�����ںϽ�����Լ������ȷ���Ӧ�ù㷺����ش��������⣺
(1)Niԭ�ӵĺ�������Ų�ʽΪ______________________________��
(2)Ni0��Fe0�ľ���ṹ���;����Ȼ��Ƶ���ͬ��Ni2+��Fe2+�����Ӱ뾶�ֱ�Ϊ69 pm��78 pm�����۵�NiO ________ FeO(�<����>��)��
(3)Ni0������Ni��O����λ���ֱ�Ϊ_______________��_______________��
(4)����������(La)�γɵĺϽ���һ�����õĴ�����ϣ��侧���ṹʾ��ͼ������ͼ��ʾ���úϽ�Ļ�ѧʽΪ_______________��


(5)����ͪ뿳����ڼ���Ni2+����ϡ��ˮ�����У�����ͪ���Ni2+��Ӧ�������ʺ�ɫ��������ṹ������ͼ��ʾ��
�ٸýṹ�У�̼̼֮��Ĺ��ۼ�������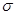 ����̼��֮��Ĺ��ۼ�������______________������֮���γɵĻ�ѧ����_______________��
����̼��֮��Ĺ��ۼ�������______________������֮���γɵĻ�ѧ����_______________��
�ڸýṹ�У�����֮������ۼ���ɴ���_______________��
�۸ýṹ�У�̼ԭ�ӵ��ӻ����������_______________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com