
����Ŀ������ͼ��ʾװ���н��а��Ĵ�����ʵ�飺������ƿ�ڵ�Ũ��ˮ�в���ͨ������������ȵIJ�˿����ƿ�в��ӽ�Һ�档��Ӧ�����У��ɹ۲쵽ƿ���к���ɫ�����������˿ʼ�ձ��ֺ��ȡ������й�˵���������(����)

A. ��Ӧ����Һ�к���NO3-
B. ��Ӧ����Һ��c(H��)����
C. ʵ��������л��Ϸ�Ӧ����
D. ʵ�������NH3H2O�ĵ��볣�������ܷ����仯
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijʵ��С�����ü����ƣ�HCOONa���Ʊ�Na2S2O4���ⶨ��Ʒ�Ĵ��ȣ�ʵ��װ�ã��г֡����������ԣ���ͼ

����ӦҺ�����ᴿ�õ�Na2S2O4��Ʒ��ȡ��Ʒmg����ˮ��������Ϊ100mL��ȡ25��00mL������ƿ�У�����NaOH��Һ��ָʾ������cmol��mol-1��K3[Fe(CN)6]����Һ���еζ�{4K3[Fe(CN)6]+2Na2S2O4+8NaOH=3K4[Fe(CN)6]+4Na2SO3+Na4[Fe(CN)6]+4H2O}���ζ����յ�ʱ�����ı�ҺVmL�����Ʒ�Ĵ���Ϊ________��д������ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)ij�л���A�Ľṹ��ʽ����ͼ���밴Ҫ��д����Ӧ����Ľṹ��ʽ
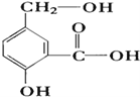
��A��NaOH��Һ��Ӧ ____________________________��
��A��NaHCO3��Һ��Ӧ _________________________________��
��A��Na��Ӧ _______________________��
(2)���������һ�����ϣ�����������ķ����ϳɣ�![]()
��д��A��B��C��D�Ľṹ��ʽ��
A ____________B_____________C_____________D_______________
��D�кܶ�ͬ���칹�壬���к����Ȼ���ͬ���칹���У������ϵ�һ�ȴ�����2�ֵĽṹ��ʽΪ________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CH3COOH��H2SO3����ѧ��ѧ�г��������ᣬ��ش��������⣺
��1�������£�����������֤��CH3COOHΪ������_________��
a�� CH3COONa ��Һ��pH����7��
b����ͬ�����ͬŨ�ȵ�����ʹ�����Һ���ֱ�������п�۷�Ӧ���ų���ͬ�����������
c����ͬ�������ͬpH������ʹ��ᣬ�����к�NaOH�����ʵ����ࣻ
d��0.01mol/L��CH3COOH��Һ��PH > 2
��2�������£���0.1 mol��L��1 CH3COOH��Һ�м�����ˮϡ�ͣ�ϡ�ͺ����и����������_________��
a.![]() b.
b.![]() c.c(OH-)
c.c(OH-)
��3����Ũ�Ⱦ�Ϊ0.1mol/LCH3COOH��CH3COONa��Һ�������ϣ���û��Һ��c(CH3COO��)>c(Na+)�������й�ϵʽ����ȷ������_____��
A��c(H+) > c(OH��) B��c(H+) < c(OH��)
C��c(CH3COOH) > c(CH3COO��) D��c(CH3COOH) + c(CH3COO��)=0.1mol/L
��4��������������ʵ���Ũ�ȵĴ��������������Һ��ϣ���Һ��_______������������������������������������ԭ���� _______________�������ӷ���ʽ��ʾ��������pH��3�Ĵ����pH��11������������Һ�������Ϻ���Һ��__________��������������������������������������Һ��c(Na��) ___________c(CH3COO��) ������ ���� ���������������� ����
��5��25��ʱ��H2SO3�ĵ��볣��Ka1=1��10-2mol/L��Ka2=6��10-3mol/L������¶���NaHSO3��ˮ��ƽ�ⳣ��Kh=__________mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����ȼ�ϵ�ؾƾ������ԭ����ͼ��ʾ������˵������ȷ���ǣ� ��

A.�����Ϸ����ķ�Ӧ�ǣ�O2��4e-��2H2O===4OH-
B.�����Ϸ����ķ�Ӧ�ǣ�CH3CH2OH��4e-��H2O ===CH3COOH��4H+
C.���ʱ���������Һ�е�H+���ƶ�
D.����0.4 mol����ת�ƣ������ı�״����4.48 LO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼʾ���Ӧ�������������
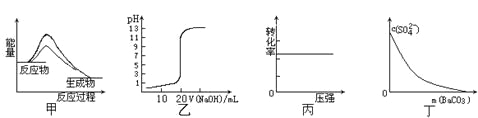
A.ͼ�ױ�ʾʹ�ô����÷�Ӧ����H��С
B.ͼ�ұ�ʾ0��10mol��L-1NaOH��Һ�ζ�20��00mL0��10mol��L-1CH3COOH��Һ���õ��ĵζ�����
C.ͼ����ʾ��ӦH2(g) + I2(g)![]() 2HI(g)��H2��ת������ѹǿ�ı仯
2HI(g)��H2��ת������ѹǿ�ı仯
D.ͼ����ʾ�ڱ���Na2SO4��Һ����BaCO3�������Һ��c(SO42-)��Ũ�ȱ仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ӧ�ù㷺�Ľ�������������(��Ҫ�ɷ�ΪAl2O3����SiO2��Fe2O3������)Ϊԭ���Ʊ�����һ�ֹ����������£�

ע��SiO2����������ʱת��Ϊ�������Ƴ�����
��1����������ʱ����ƫ�����Ƶ����ӷ���ʽΪ_____________________��
��2������������������Һ�м���NaHCO3��Һ����Һ��pH_________ (��������������������������С��)��
��3����������ǵ������Al2O3������������������ʯī�����ģ�ԭ����___________��
��4����������ǵ��Na2CO3��Һ��ԭ����ͼ��ʾ�������ĵ缫��ӦʽΪ_____________________����������������A�Ļ�ѧʽΪ____________��

��5��������1000��ʱ����N2��Ӧ�Ʊ�AlN������������������NH4Cl���岢��ֻ�ϣ�������AlN���Ʊ�������Ҫԭ����_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��H(3-��-5-���������������)����Ҫ���л����м��壬������A(C7H8)ͨ����ͼ·�ߺϳɡ�

��ش��������⣺
(1)C�Ļ�ѧ����Ϊ________��G�������Ĺ��������Ѽ���_______(������)��
(2)B�Ľṹ��ʽΪ________��B����C�ķ�Ӧ����Ϊ___________��
(3)��G����H�Ļ�ѧ����ʽΪ___________��
(4)������F��ͬ���칹������ͬʱ�������������Ĺ���________�֡�
�ٰ������ǻ�ֱ�����ڱ����� �ڱ�����������ȡ�������ܷ���ˮ�ⷴӦ
(5)����ö������ұ�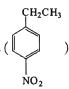 Ϊ��ʼԭ���Ʊ�������
Ϊ��ʼԭ���Ʊ�������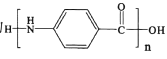 �ĺϳ�·�ߣ������Լ���ѡ����_________________________
�ĺϳ�·�ߣ������Լ���ѡ����_________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ���Ȼ���![]() Ϊ��ɫ��б���壬���׳�����������������������εĺϳ�ԭ�ϡ�ij����С������
Ϊ��ɫ��б���壬���׳�����������������������εĺϳ�ԭ�ϡ�ij����С������![]() dz��ɫ��ĩ
dz��ɫ��ĩ![]() ��ľ̿�ۺ�
��ľ̿�ۺ�![]() �Ʊ���ˮ
�Ʊ���ˮ![]() ���ⶨ�䴿�ȣ��Ʊ�ʱ�õ���ʵ��װ�����¡�
���ⶨ�䴿�ȣ��Ʊ�ʱ�õ���ʵ��װ�����¡�
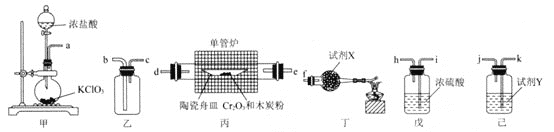
��ش��������⣺
![]() ����װ�õ�����˳��Ϊ
����װ�õ�����˳��Ϊ![]() ________
________![]() ��װ�ýӿڴ���Сд��ĸ
��װ�ýӿڴ���Сд��ĸ![]() ��
��
![]() �Լ�X��Y�ɷֱ�ѡ�������Լ��е�________��________
�Լ�X��Y�ɷֱ�ѡ�������Լ��е�________��________![]() �����
�����![]() ��װ�ö��оƾ��Ƶ�������________��
��װ�ö��оƾ��Ƶ�������________��
![]() ��Һ
��Һ ![]() ��ˮ
��ˮ![]()
![]() ��ʯ��
��ʯ�� ![]() ����
����![]() ��Һ
��Һ ![]() ����ʳ��ˮ
����ʳ��ˮ
![]() װ������ʹ�ôֵ��ܵ�ԭ��Ϊ________��
װ������ʹ�ôֵ��ܵ�ԭ��Ϊ________��
![]() װ�ñ��з�����Ӧ�Ļ�ѧ����ʽΪ________��
װ�ñ��з�����Ӧ�Ļ�ѧ����ʽΪ________��
![]() ʵ�������װ�����е�ʵ������Ϊ________��
ʵ�������װ�����е�ʵ������Ϊ________��
![]() �ⶨ��Ʒ�����Ȼ����ĺ�����
�ⶨ��Ʒ�����Ȼ����ĺ�����
��ȡ![]() ��Ʒ����ˮ���Ƴ�
��Ʒ����ˮ���Ƴ�![]() ��Һ��ȡ
��Һ��ȡ![]() ������Һ����ƿ�У�����
������Һ����ƿ�У�����![]() ��
��![]() ��Һ����ַ�Ӧ����ָʾ������
��Һ����ַ�Ӧ����ָʾ������![]() ��
��![]() ����Һ�ζ����յ�ʱ�����ı���Һ�����Ϊ
����Һ�ζ����յ�ʱ�����ı���Һ�����Ϊ![]() ��֪��
��֪��![]() ��
��![]() ��
��
![]() ����Ʒ��
����Ʒ��![]() ����������Ϊ________
����������Ϊ________![]() ��������ȷ��
��������ȷ��![]() ��
��
![]() ���ζ����յ�ʱ�����ֵζ��ܼ��촦�����ݣ���ⶨ���________
���ζ����յ�ʱ�����ֵζ��ܼ��촦�����ݣ���ⶨ���________![]() ����ƫ������ƫ����������Ӱ����
����ƫ������ƫ����������Ӱ����![]() ��
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com