
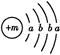
 ,X����������Y�������ӵĵ��Ӳ�ṹ��ͬ��Ԫ��Z��W��Ϊ������Ԫ��,����ԭ�ӵ�������������������Ӳ�����2��,Z��Y������Z��W���γ�һ��WZ2�ͷ��ӡ�
,X����������Y�������ӵĵ��Ӳ�ṹ��ͬ��Ԫ��Z��W��Ϊ������Ԫ��,����ԭ�ӵ�������������������Ӳ�����2��,Z��Y������Z��W���γ�һ��WZ2�ͷ��ӡ�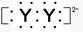
 2Y-+Z��
2Y-+Z��
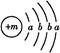 ������֪��Ϊ
������֪��Ϊ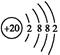 ,��Ԫ��ΪCa,����CaY2��֪YΪ-1��,����Y-��Ca2+�ĵ��Ӳ�ṹ��ͬ,��֪YΪCl��(2)Z��Wԭ�ӵ�����������������Ӳ�����2��,��Z��W���γ�һ��ZW2�ͷ���,��Z��W��C��S��Z��Y����,��Z��S,W��C��(3)CaCl2�ǽ������Ӽ������ӻ�����,CS2�ǽ������Լ��Ĺ��ۻ�����,A��,B��;S�ķǽ���������Cl,��H2S���ȶ��Ա�HCl��,C��;Ca2+��Cl-������ͬ�ĵ��Ӳ�ṹ,��Ca2+�ĺ˵������,�뾶С,D����(4)XY2ΪCaCl2,�����ʽΪ
,��Ԫ��ΪCa,����CaY2��֪YΪ-1��,����Y-��Ca2+�ĵ��Ӳ�ṹ��ͬ,��֪YΪCl��(2)Z��Wԭ�ӵ�����������������Ӳ�����2��,��Z��W���γ�һ��ZW2�ͷ���,��Z��W��C��S��Z��Y����,��Z��S,W��C��(3)CaCl2�ǽ������Ӽ������ӻ�����,CS2�ǽ������Լ��Ĺ��ۻ�����,A��,B��;S�ķǽ���������Cl,��H2S���ȶ��Ա�HCl��,C��;Ca2+��Cl-������ͬ�ĵ��Ӳ�ṹ,��Ca2+�ĺ˵������,�뾶С,D����(4)XY2ΪCaCl2,�����ʽΪ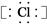 -Ca2+
-Ca2+ -,WZ2ΪCS2,�ṹͬCO2,�ṹʽΪS
-,WZ2ΪCS2,�ṹͬCO2,�ṹʽΪS C
C S,H2SΪ����,Ӧд�ɷ���ʽ,��Cl2+H2S
S,H2SΪ����,Ӧд�ɷ���ʽ,��Cl2+H2S 2H++2Cl-+S��,A��C����
2H++2Cl-+S��,A��C����


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A�� ��A��N��m�� mol ��A��N��m�� mol | B�� ��A��N�� mol ��A��N�� mol |
C�� ��A��N�� mol ��A��N�� mol | D�� ��A��N��m�� mol ��A��N��m�� mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
|
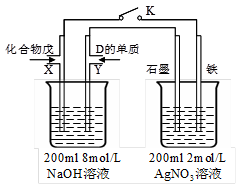
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ,1��
,1�� �������
������� ��
�� ��˵������ȷ����(����)
��˵������ȷ����(����)| A�������ֺ��� | B����Ϊͬλ�� | C���������ֱ�Ϊ176��177 | D�����������1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| Ԫ������ | Ԫ�ر�� | ||||||
| �� | �� | �� | �� | �� | �� | �� | |
| ԭ�Ӱ뾶(nm) | 0.073 | 0.075 | 0.152 | 0.110 | 0.099 | 0.186 | 0.143 |
| ��������ϼ� | �� | ��5 | ��1 | ��5 | ��7 | ��1 | ��3 |
| ������ϼ� | ��2 | ��3 | �� | ��3 | ��1 | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Ԫ�����ڱ��дӢ�B�嵽��B��ʮ�����е�Ԫ�ض��ǽ���Ԫ�� |
| B�����ԣ�NaOH > NH3��H2O ,����Ԫ�صĽ����ԣ�Na > N |
| C��ͬ���ڵڢ�A����ڢ�A���Ԫ��ԭ������֮�һ��Ϊ1 |
| D���ڢ�A��Ԫ�ش��ϵ��£����⻯����ȶ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com