
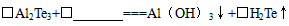


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| Ԫ�ش��� | �����Ϣ |
| T | T�ĵ���������ˮ���ҷ�Ӧ������ǿ������Һ�к������ֵ�������ͬ������������ |
| X | X��ԭ�����������������ڲ������������ |
| Y | �ڵ������ڽ���Ԫ���У�Y�ļ����Ӱ뾶��С |
| Z | T��X��Z��ɵ�36���ӵĻ�����A�Ǽ�������������Ҫ�ɷ� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ��ˮ��ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��11�֣�T��X��Y��Z��Q��R��WΪ���ڱ�ǰ������Ԫ�أ�ԭ���������ε���������ijЩԪ�ص������Ϣ���±���
| Ԫ�� | �����Ϣ |
| T | Tԭ�����������������������ֱ�����ԭ��������� |
| X | X�Ļ�̬ԭ���е���ռ������������ͬ��ԭ�ӹ������ÿ�ֹ���еĵ�������ͬ |
| Z | Z�Ļ�̬ԭ�Ӽ۵����Ų�Ϊ |
| Q | �ڸ�Ԫ�����������У�Q�Ļ�̬ԭ�ӵĵ�һ��������С |
| R | 3p�ܼ�����1������ |
| W | W��һ�ֺ��ص�������Ϊ65��������Ϊ36 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ӱ�ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ��ƶ���
��11�֣�T��X��Y��Z��Q��R��WΪ���ڱ�ǰ������Ԫ�أ�ԭ���������ε���������ijЩԪ�ص������Ϣ���±���
|
Ԫ�� |
�����Ϣ |
|
T |
Tԭ�����������������������ֱ�����ԭ��������� |
|
X |
X�Ļ�̬ԭ���е���ռ������������ͬ��ԭ�ӹ������ÿ�ֹ���еĵ�������ͬ |
|
Z |
Z�Ļ�̬ԭ�Ӽ۵����Ų�Ϊ |
|
Q |
�ڸ�Ԫ�����������У�Q�Ļ�̬ԭ�ӵĵ�һ��������С |
|
R |
3p�ܼ�����1������ |
|
W |
W��һ�ֺ��ص�������Ϊ65��������Ϊ36 |
��1��X��Y��Q����Ԫ�صĵ縺���ɴ�С��˳���� ����Ԫ�ط��ű�ʾ����
��2��X��Yԭ�ӽ���γɵ�X3Y4���壬����ṹ����ʯ���ƣ���X3Y4������۵�Ƚ��ʯҪ ����ߡ������͡�����
��3��W2+�ĺ�������Ų�ʽΪ ��Ԫ��W������������е������Լ�������Ӧ�����ɳ����W��HCl��O2��WCl��HO2��HO2 �������ᣩ������һ���������Ҳ��һ�����ɻ������м��ߵĻ��ԡ�����˵�����ʾ�������
A����������O2 B��HO2�ڼ��в����ȶ�����
C������������HO2 D��1 molW�μӷ�Ӧ��1 mol���ӷ���ת��
��4��X��Y��Z�ֱ�����Ԫ�ؿ��Թ���A��B��C��D�ȶ������ӡ�����A��B��C��Ϊ10��������DΪ18��������AΪ5ԭ�Ӻ˵�+1�������ӣ���A+������ԭ���ӻ���ʽΪ_______. BΪ4ԭ�Ӻ˵�+1�������ӣ���B+����ʽΪ___________��CΪ4��ԭ�Ӻ˹��ɵķ��ӣ�����C��Ϊ�ȵ�����ķ��ӿ�����_______��д�ṹʽ����D��������Ԫ�ص�ԭ�Ӹ���֮��Ϊ1:1����DΪ ������ԡ��Ǽ��ԡ������ӡ�ij˫ԭ�ӵ��ʷ���EҲΪ18��������E��ˮ�ķ�Ӧ�Ļ�ѧ����ʽΪ______________________��
��5����֪25�桢101 kPa�����£�
4R��s��+3Z2��g�� 2R2Z3��s�� ��H=-2835��9 kJ��mol
4R��s��+2Z3��g�� 2R2Z3��s�� ��H=-3119��1 kJ��mol
��16g Z2��g����ȫת��ΪZ3��g���ġ�H= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±�������Ԫ�ص��������
| ����\Ԫ�� | 8O | 16S | 34Se | 52Te |
| �����۵㣨�棩 | �D218.4 | 113 | 450 | |
| ���ʷе㣨�棩 | �D183 | 444.6 | 685 | 1390 |
| ��Ҫ���ϼ� | �D2 | �D2��+4��+6 | �D2��+4��+6 | |
| ԭ�Ӱ뾶 | ������ | |||
| ������H2��Ӧ��� | ��ȼʱ���� | ���Ȼ��� | �����ѻ��� | ����ֱ�ӻ��� |
����ݱ��ش��������⣺
��1�������۵���Χ������
��2���������н�ǿ�� ��������ԡ���ԭ�ԡ�������˷��ڿ����г��ڱ����ױ��ʣ�����ܷ����ڻ�ѧ��Ӧ����ʽΪ ��
��3����ҵ��Al2Te3�������Ʊ�H2Te��������л�ѧ����ʽ��
��Al2Te3+�� ===Al��OH��3��+��H2Te��
��4����֪�ڳ����£�H2��S��Ӧ����17gH2S�ų�56.1KJ����������д������ֽ���Ȼ�ѧ����ʽ�� ��
��5����ͼ��ʾΪ����Ԫ�ص�����H2��Ӧ�����е�
�����仯ʾ��ͼ��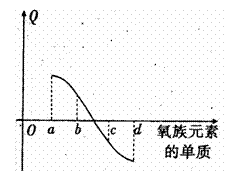 ����a��b��c��d������ʾ
����a��b��c��d������ʾ
������ijһԪ�صĵ��ʣ�QΪ��ͬ���ʵ���
�ĵ�����H2��Ӧ�ų�����������
a���� ��c����
����������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com