


 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
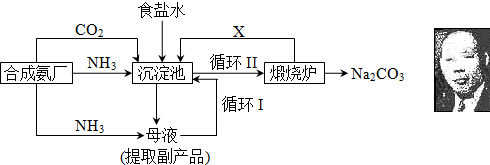
��16�֣��ҹ���ѧ��°���ͼ���ĸ����Ĵ����������գ��������̿ɼ�Ҫ��ʾ���£�
���������������ڲ�ͬ�¶��µ��ܽ�ȣ�g / 100 gˮ����
|
| 0�� | 10�� | 20�� | 30�� | 40�� | 50�� | 60�� | 100�� |
| NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
| NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | ���� | �� | �� | �� |
| NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | �� |
| NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
�٣�35��NH4HCO3���зֽ�
��ش�
��1���������з�Ӧ�¶ȿ�����30�桫35�棬����Ϊ������35�棬�� ��������30�棬�� ��Ϊ���ƴ��¶ȷ�Χ����ȡ�ļ��ȷ���Ϊ ��
(2) �������з����Ļ�ѧ��Ӧ����ʽ�� ��
��3������Ʒ��һ����;Ϊ ��д������������X���ʵķ���ʽ ��
��4��������Ϻ�������30���ӣ�Ŀ���� �����ú�ֻ����NaHCO3�����ԭ���� ��������ˮϴ��NaHCO3�����Ŀ���dz�ȥ ���ʣ��Ի�ѧʽ��ʾ����
(5) Ϊ�����Ʒ̼�������Ƿ����Ȼ��ƣ���ȡ������������ˮ���ٵμ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��㶫ʡ÷����ѧ�����ڶ����¿������ۺ����⣨��ѧ���֣� ���ͣ�ʵ����
��16�֣��ҹ���ѧ��°���ͼ���ĸ����Ĵ����������գ��������̿ɼ�Ҫ��ʾ���£�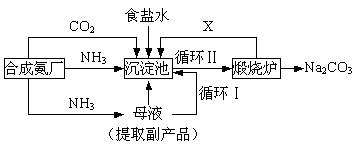

���������������ڲ�ͬ�¶��µ��ܽ�ȣ�g / 100 gˮ����
 | 0�� | 10�� | 20�� | 30�� | 40�� | 50�� | 60�� | 100�� |
| NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
| NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | ���� | �� | �� | �� |
| NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | �� |
| NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ�����ڶ����¿������ۺ����⣨��ѧ���֣� ���ͣ�ʵ����
��16�֣��ҹ���ѧ��°���ͼ���ĸ����Ĵ����������գ��������̿ɼ�Ҫ��ʾ���£�
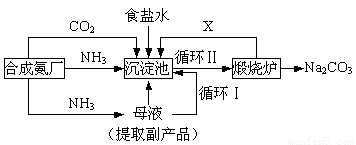

���������������ڲ�ͬ�¶��µ��ܽ�ȣ�g / 100 gˮ����
|
0�� |
10�� |
20�� |
30�� |
40�� |
50�� |
60�� |
100�� |
|
|
NaCl |
35.7 |
35.8 |
36.0 |
36.3 |
36.6 |
37.0 |
37.3 |
39.8 |
|
NH4HCO3 |
11.9 |
15.8 |
21.0 |
27.0 |
���� |
�� |
�� |
�� |
|
NaHCO3 |
6.9 |
8.1 |
9.6 |
11.1 |
12.7 |
14.5 |
16.4 |
�� |
|
NH4Cl |
29.4 |
33.3 |
37.2 |
41.4 |
45.8 |
50.4 |
55.3 |
77.3 |
�٣�35��NH4HCO3���зֽ�
��ش�
��1���������з�Ӧ�¶ȿ�����30�桫35�棬����Ϊ������35�棬�� ��������30�棬�� ��Ϊ���ƴ��¶ȷ�Χ����ȡ�ļ��ȷ���Ϊ ��
(2) �������з����Ļ�ѧ��Ӧ����ʽ�� ��
��3������Ʒ��һ����;Ϊ ��д������������X���ʵķ���ʽ ��
��4��������Ϻ�������30���ӣ�Ŀ���� �����ú�ֻ����NaHCO3�����ԭ���� ��������ˮϴ��NaHCO3�����Ŀ���dz�ȥ ���ʣ��Ի�ѧʽ��ʾ����
(5) Ϊ�����Ʒ̼�������Ƿ����Ȼ��ƣ���ȡ������������ˮ���ٵμ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
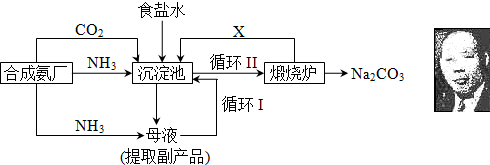
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com