
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| O | - 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
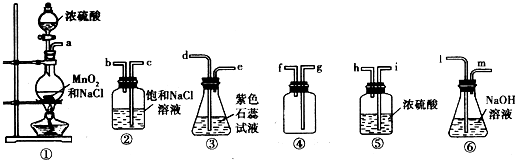
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
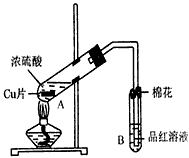
 ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
| ||
| ||
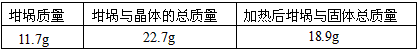
| �������� | �����뾧��������� | ���Ⱥ���������������� |
| 11.7g | 22.7g | 18.9g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
��1��д���Թ�B�е�ʵ������ ��
��2��д��A�з�Ӧ�Ļ�ѧ����ʽ�� ��
 ��3����ַ�Ӧ����A�Թ�����ͭƬʣ�࣬������A�м���
��3����ַ�Ӧ����A�Թ�����ͭƬʣ�࣬������A�м���
NaNO3������ͭƬ�ܽ⣬��Ӧ�����ӷ���ʽ
Ϊ ��
��4����ַ�Ӧ����A�Թ�����ͭƬʣ�࣬
�����ְ�ɫ���ǣ��ð�ɫ������ ��
����ȷ�ϸð�ɫ������ʲô���ʵ�ʵ���������
�� ��
��5��B�Թܿڵ���Ӧմ�е��Լ��� ��
��6��С���Ա��4����Ӧ�����Һ�м�������������ͭ��ʹʣ�������ȫ��ת��Ϊ����ͭ�����˺���Һ����Ũ������ȴ��ᾧ�Ƶ�����ͭ���壨CuSO4��xH2O����С���Ա���ü��ȷ��ⶨ�þ�����ᾧˮx��ֵ
�������ǵ�ʵ������У����ٳ����ĴΣ�������γ�����Ŀ����
������������һ��ʵ������ݣ�
| �������� | �����뾧��������� | ���Ⱥ���������������� |
| 11.0g | 37.8g | 27.0g |
�����ϱ����ݼ����ж�x��ʵ��ֵ������ֵ��x��5�� ���ƫ����ƫС������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com