
| ������ | K+��Mg2��Fe3+��Al3+ |
| ������ | Cl-�� �� �� |
 ��=0.2 mol/L
��=0.2 mol/L ��
��
 =0.02mol��CO32-����Ũ��=
=0.02mol��CO32-����Ũ��= =0.2mol/L����A��ȷ��
=0.2mol/L����A��ȷ��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2013?����ģ�⣩ij��Һ�У�ֻ���ܺ������������еļ��֣���ÿ��ȡ100.00mL��Һ����ʵ�飺�ٵ�һ�ݼ�����������Һ�г����������ڵڶ��ݼ��������Ȼ�����Һ��ø������6.27g����������������ϴ�ӣ������ʣ��2.33g������˵��������ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ����ʦ���С��ٴ�һ�и���12��������ѧ�Ծ����������� ���ͣ���ѡ��
ij��Һ�У�ֻ���ܺ������������еļ��֣�
| ������ | K+��Mg2��Fe3+��Al3+ |
| ������ |  �� �� �� ��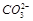 |
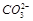 ��="0.2" mol/L B��C(K+)һ��Ϊ0.6mol/L
��="0.2" mol/L B��C(K+)һ��Ϊ0.6mol/L  ��
��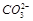
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ���ٴ�һ�и���12��������ѧ�Ծ��������棩 ���ͣ�ѡ����
ij��Һ�У�ֻ���ܺ������������еļ��֣�
|
������ |
K+��Mg2��Fe3+��Al3+ |
|
������ |
|
��ÿ��ȡ100.00mL��Һ����ʵ�飺�ٵ�һ�ݼ�����������Һ�г����������ڵڶ��ݼ��������Ȼ�����Һ��ø������6.27g����������������ϴ�ӣ������ʣ��2.33g������˵��������ǣ� ��
A��C�� ��=0.2 mol/L B��C(K+)һ��Ϊ0.6mol/L
��=0.2 mol/L B��C(K+)һ��Ϊ0.6mol/L
C�������ӿ��ܴ��� D��һ����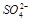 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ģ�� ���ͣ���ѡ��
| ������ | K+��Mg2��Fe3+��Al3+ | ||||
| ������ | Cl-��S
|
A��C��C
| B��C��K+��һ��Ϊ0.6mol/L | ||||
| C�������ӿ��ܴ��� | D��һ����S
|
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com