
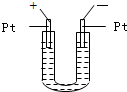
��6�֣���ͼ��ʾ��U�ιܵ���˱�ˮ�ͽ�������м���������������Ϊ1��4���Ļ�����壬�ٶ�������ˮ�е��ܽ�ȿ��Ժ��ԡ�������м���������Ļ�������װ��
�������й����ĵط����û�����建���ط�Ӧһ��ʱ�䡣
(1)�����������ķ�Ӧ���� ��Ӧ����Ӧ��õ��������л�����Ľṹ��ʽΪ ��
(2)��������Сʱ�ķ�Ӧ��U�ι��Ҷ˵�ˮ���仯��_______ ��
A�����ߡ��� B�����͡��� C�����䡡�� D����ȷ��
��6�֣�CO��CH4��Ϊ�����Ŀ�ȼ�����塣
(1)��֪��101 kPaʱ�� CH4��ȫȼ������1molҺ̬ˮ���ų�������ΪQkJ����CH4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ�ǣ� ��
(2)120�桢101 kPa�£�a mL��CO��CH4��ɵĻ��������bmL O2����ȫȼ�պָ���ԭ�¶Ⱥ�ѹǿ��
�����������������ǡ����ȫ��Ӧ������bmL������̼�����������һ����̼�ͼ�������ʵ���֮��Ϊ�� ��
����ȼ�պ����������С��a/4 mL����a��b��ϵ����ѧ��ʾʽ�� ��
��.(1)ȡ�� CH3Cl CH2Cl2 CHCl3 CCl4 (2)B
��. (1) CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H=��2QkJ��mol��1
(2)�� 2:1 ��b��5a/4
����:�������������ȡ����Ӧ���õ�������ȴ��������٣�ѹǿ��С��U�ι������ҽ���
CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H=��2QkJ��mol��1
2CO-----O2---------2CO2 CH4--------2O2-------CO2
2X X 2X Y 2Y Y
2X+Y=a x+2y=b x= (2a-b ) ��3 y=(2b-a ) ��3


 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2006?�ϳ�һģ����ͼ��ʾ��U�ι���װ�뺬����ɫʯ���Na2SO4��Һ��ֱͨ���磬һ��ʱ���U�ι��ڻ��γ���ɫ���ʺ硱����������������ɫ�Ĵ����ǣ�������
��2006?�ϳ�һģ����ͼ��ʾ��U�ι���װ�뺬����ɫʯ���Na2SO4��Һ��ֱͨ���磬һ��ʱ���U�ι��ڻ��γ���ɫ���ʺ硱����������������ɫ�Ĵ����ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
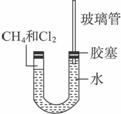
(1)���������������Ӧ��֣���ֻ����һ���л����д����ѧ����ʽ__________________��
(2)��������Сʱ�ķ�Ӧ��U�ι��Ҷ˵IJ�������ˮ���仯��___________��
A.���� B.���� C.���� D.��ȷ��
(3)U�ι���˵������仯��___________��
A.������� B.�����С C.��ʧ D.����
(4)�Խ���U�ι��Ҷ˵IJ�������ˮ���仯��ԭ��
_____________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�Ž�һ�и�һ��ѧ�����п������ƻ�ѧ�Ծ����������� ���ͣ�ʵ����
��6�֣���ͼ��ʾ��U�ιܵ���˱�ˮ�ͽ�������м��������(�����Ϊ1��4)�Ļ�����壬�ٶ�������ˮ�е��ܽ�ȿ��Ժ��ԡ�������м���������Ļ�������װ�÷������й����ĵط����û�����建���ط�Ӧһ��ʱ�䡣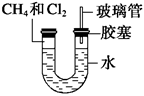
(1)���������������Ӧ��֣���ֻ����һ���л��
��д����ѧ����ʽ��
_____________________________________________________��
(2)����Ŀ�м����������������Ϊ1��1����õ��IJ���Ϊ________��
A��CH3Cl��HCl
B��CCl4��HCl
C��CH3Cl��CH2Cl2
D��CH3Cl��CH2Cl2��CHCl3��CCl4��HCl
(3)��������Сʱ�ķ�Ӧ��U�ι��Ҷ˵�ˮ���仯��
________________________________________________________________________��
A������ B������ C������ D����ȷ��
(4)��ˮ�к���Na2SiO3������U�ι���˻�۲쵽
________________________________________________________________________��
(5)�Ҷ˲����ܵ�������______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�츣��ʡ����У�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ͼ��ʾ��U�ι���װ�뺬����ɫʯ���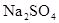 ��Һ��ֱͨ���磬һ��ʱ���U�ι��ڻ��γ���ɫ���ʺ硱����������������ɫ�Ĵ����ǣ���
��
��Һ��ֱͨ���磬һ��ʱ���U�ι��ڻ��γ���ɫ���ʺ硱����������������ɫ�Ĵ����ǣ���
��
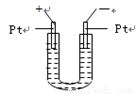
A�������ϡ���
B���졢������
C���졢�ϡ���
D�������졢��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���������ʷֱ����װ��ˮ����ƿ��,����������U�ιܵ�����,��
֪U�ι���Ԥ��װ������ˮ(Ϊʹˮ���� ��,Ԥ��Ⱦ�ɺ�ɫ)����ͼ��ʾ,
���U�ι� ���Һ������,���������ʿ�����(��)
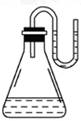
A.NaOH���塡������B.ŨH2SO4 C.NH4NO3���塡���� D.Na2O2����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com