
��֪Ba(AlO2)2������ˮ����ͼ��ʾ������Al2(SO4)3��Һ����μ���Ba(OH)2 ��Һʱ�����ɳ��������ʵ���y�����Ba(OH)2�����ʵ���x�Ĺ�ϵ�������й�������ȷ����
��Һʱ�����ɳ��������ʵ���y�����Ba(OH)2�����ʵ���x�Ĺ�ϵ�������й�������ȷ����
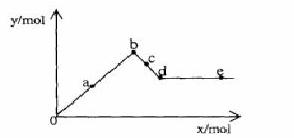
A.a��bʱ���������ʵ�����Al(OH)3��BaSO4��
B.c��dʱ��Һ�����ӵ����ʵ�����AlO2-��Ba2+��
C.a��dʱ���������ʵ�����BaSO4����С��Al(OH)3
D.d��eʱ��Һ�����ӵ����ʵ�����Ba2+���ܵ���OH-
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���÷Ͼɶ�п��Ƥ���Ʊ�����Fe3O4�������Ӽ�������ZnO���Ʊ��������£�

��֪��Zn���仯�����������Al���仯������������ơ���ش��������⣺
��1����NaOH��Һ�����Ͼɶ�п��Ƥ�������� ��
A��ȥ������ B���ܽ��п�� C��ȥ������ D���ۻ�
��2��������ҺA��pH�ɲ���Zn��OH��2������Ϊ�Ƶ�ZnO���������������� ��
��3������ҺB�Ƶ�Fe3O4�������ӵĹ����У������ͨ��N2����ԭ���� ��
��4��Fe3O4���������ܷ��ü�ѹ���˷�ʵ�ֹ�Һ���룿 ����ܡ����ܡ����������� ��
��5�����ظ���ط���һ��������ԭ�ζ������ɲⶨ����Fe3O4�еĶ�������������������Ũ��Ϊ0.01000mol��L-1��K2Cr2O7����Һ250mL��Ӧȷ��ȡK2Cr2O7 g������4λ��Ч���֣���֪MK2Cr2O7=294.0g��mol-1�������Ƹñ���Һʱ��������������Ҫ�õ����� �����ñ�ű�ʾ��
�ٵ�����ƽ ���ձ� ����Ͳ �ܲ����� ������ƿ ��ͷ�ι� ����Һ��
��6���ζ������У�����ζ�ǰװ��K2Cr2O7����Һ�ĵζ��ܼ��첿�������ݣ����ζ�������������ʧ����ζ������ ���ƫ����ƫС�����䡱����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������Ͻ��������г��õIJ��ϣ�����˵����ȷ���ǣ� ��
A.����������Ͻ�ֻ������Ԫ��
B.һ�������£����ۿ���ˮ������Ӧ
C.�������ᷴӦ�����Ͻ������ᷴӦ
D.�ڿ����У�����ȶ�п�������ʴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йؽ��������仯�����������ȷ����
 A.���ڳ����²�����������Ӧ
A.���ڳ����²�����������Ӧ
B.��������������Ӧ
C.�����������ᣬ�������ڼ�
D.������ֻ�����ᷴӦ��������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
.�������Ļ�����Ӧ�ù㷺.��FeCl3������������ӡˢ��·ͭ�帯ʴ��������ֹѪ���ȡ�
(1)д��FeCl3��Һ��ʴӡˢ��·ͭ������ӷ���ʽ_______________________________��
(2)����(1)�еķ�Ӧ��Ƴ�ԭ��أ��뻭��ԭ��ص�װ��ͼ�����������������д���缫��Ӧʽ��
������Ӧ___________________ ������Ӧ________________________
(3)��ʴͭ���Ļ����Һ�У���Cu2+��Fe3+��Fe2+��Ũ�Ⱦ�Ϊ0.10mol��L-1��������±����������ݺ�ҩƷ��������ȥCuCl2��Һ��Fe3+��Fe2+��ʵ�鲽��__________________________��
| �������↑ʼ����ʱ��pH | �������������ȫʱԼpH | |
| Fe3+ Fe2+ Cu2+ | 1.9 7.0 4.7 | 3.2 9.0 6.7 |
| �ṩ��ҩƷ��Cl2 ŨH2SO4 NaOH��Һ CuO Cu |
(4)ij������Ա�������ʲ���������и�ʴ����������ijЩ����Һ�и�ʴ�������ԡ�����ϱ��ṩ��ҩƷ��ѡ������(ˮ����ѡ)��������ʵ�飬��֤���ʲ�����ױ���ʴ��
�йط�Ӧ�Ļ�ѧ����ʽ__________________________
���ʲ���ָ�ʴ��ʵ������_________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һЩ������͵���֮��ɷ�����ͼ��ʾ�ķ�Ӧ��
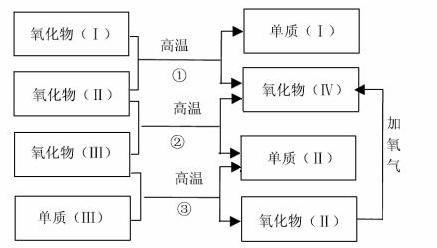
���У���������Ǻ���ɫ���塢������������������ڷ�Ӧ�����¶������塣
��1��������Ļ�ѧʽ������ʽ���� ��
������Ļ�ѧʽ������ʽ���� ��
��2����Ӧ�ٵĻ�ѧ����ʽ��
��
��Ӧ�ڵĻ�ѧ����ʽ�� ��
��Ӧ�۵Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ض�����Ҫ�Ĺ�ҵ��Ʒ����ش�
(1)��ҵұ�����Ļ�ѧ����ʽ��___________________________________��
(2)��������������Һ��Ӧ�����ӷ���ʽ��__________________________________��
(3)��ҵƷ�������ص���Һ�к���ijЩ����������ʣ��������ӽ���Ĥ������ᴿ��������װ�������ӽ���Ĥ(ֻ����������ͨ��)���乤��ԭ����ͼ��ʾ��
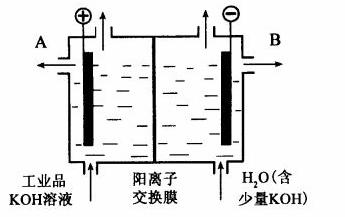
�ٸõ��۵�������Ӧʽ��__________________________��
��ͨ�翪ʼ������������ҺpH�����������ԭ��
_______________________________________________________��
�۳�ȥ���ʺ������������Һ��Һ�����______(��д��A����B��)������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ�У�����������ԭ��Ӧ���ǣ� ����
A��2NaHCO3 Na2CO3+H2O��+CO2��
Na2CO3+H2O��+CO2��
B��KClO3+6HCl (Ũ)==KCl+3H2O+3Cl2��
C��CO2+Ca(OH)==CaCO3��+H2O
D��MnO2+4HCl (Ũ) MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�Ȼ�����Ʒ����FeCl2���ʣ���Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�

��1������I���õ��IJ����������ձ����������⣬��������
(���������ƣ���
��2�����������ˮ���ɳ��������ӷ���ʽΪ ��
��3����������ˮ�����������Լ��е� ���棨����ţ���
A��H2O2 B����ˮ C��NaClO
��4��������м�������Ƿ�ϴ���ķ����� ��
��5������������ΪW1g�����������պ�Ĺ���������ΪW2g����Ʒ����Ԫ�ص���������Ϊ ����ѽ��Լ�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com