
(16�֣�����ΪԪ�����ڱ��е�һ���֣���ע�����������û�ѧ����ش��������⣺
 | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | | | | | | �� | �� | |
| 3 | �� | �� | �� | | | | | �� |
| 4 | �� | �� | | | | | | |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(16�֣�����ΪԪ�����ڱ��е�һ���֣���ע�����������û�ѧ����ش��������⣺
|
| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 |
|
|
|
|
| �� | �� |
|
| 3 | �� | �� | �� |
|
|
|
| �� |
| 4 | �� | �� |
|
|
|
|
|
|
�� 8��Ԫ���У���ѧ��������õ��� ���ǽ�������ǿ���� ��
�� �٢ڢݵ�����������Ӧˮ����ļ�����ǿ������˳��Ϊ ��
�� �ڢۢ����γɵļ����Ӱ뾶�ɴ�С��˳��Ϊ ��
�� ���⻯�ﳣ������Ԫ�آڵĹ������ﷴӦ�����ӷ���ʽΪ ��
�� �ٺ͢��γɵĵ���ɫ����ĵ���ʽΪ ��������ѧ��������Ϊ������Ӽ������ۼ����� ��
�� �ٺ͢ݵ�����������Ӧˮ�������Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ��һ��ѧ�ڵ�һ�ν��Բ��Ի�ѧ�Ծ����������� ���ͣ������
(16��)�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����
�ش��������⣺
��1���ܡ��ݡ���ԭ�Ӱ뾶�ɴ�С��˳��Ϊ_______________________���ڡ��ߡ������ۺ������������ǿ������˳����______________________��
��2���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡҺ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ�_________________��
a��MnO2������������b��FeCl 3���������� c��Na2SO3���������� d��KMnO4
����֪1 �˸�Һ̬������ֽ�ɢ۵ĵ��ʺ�һ�ֳ���Һ��ʱ���ɷų�2.9kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��
��3����ҵ�ϳ����õ��A�ͱ���ʯ(Na3AlF6)�����ķ���ұ���Ʊ��ĵ��ʣ����A��������ͺ��������������ǶȽ��ͼӱ���ʯ(Na3AlF6)��ԭ�� �� ��д�����ʱ�ĵ缫��Ӧʽ�� ��
��4���ס��ҡ�������������Ԫ����ɵ�˫ԭ�ӷ��ӻ���˫ԭ�������ӣ��Ҽס��ҡ����ĵ���������ȡ�����һ�ּ�ǿ�������Ե��ʡ�����ݵ������ӿ��γ�һ�ֵ���ɫ����B���ù����ˮ��Ӧ�ɵõ��۵ĵ��ʡ���B����ʽ ���ҵĽṹʽ �������Ԫ�ص�ԭ�ӽṹʾ��ͼ ����֤�������Ԫ�طǽ����Ժ�ǿ����ʵ ��(�ξ�һ������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭ʡ��һ��ѧ�ڵ�һ�ν��Բ��Ի�ѧ�Ծ��������棩 ���ͣ������
(16��) �±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����
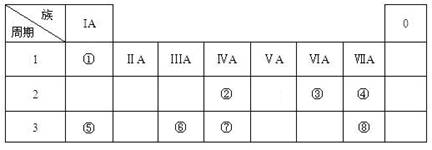
�ش��������⣺
��1���ܡ��ݡ���ԭ�Ӱ뾶�ɴ�С��˳��Ϊ_______________________���ڡ��ߡ������ۺ������������ǿ������˳����______________________��
��2���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡҺ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ�_________________��
a��MnO2������������ b��FeCl 3���������� c��Na2SO3���������� d��KMnO4
����֪1 �˸�Һ̬������ֽ�ɢ۵ĵ��ʺ�һ�ֳ���Һ��ʱ���ɷų�2.9kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��
��3����ҵ�ϳ����õ��A�ͱ���ʯ(Na3AlF6)�����ķ���ұ���Ʊ��ĵ��ʣ����A��������ͺ��������������ǶȽ��ͼӱ���ʯ(Na3AlF6)��ԭ�� �� ��д�����ʱ�ĵ缫��Ӧʽ�� ��
��4���ס��ҡ�������������Ԫ����ɵ�˫ԭ�ӷ��ӻ���˫ԭ�������ӣ��Ҽס��ҡ����ĵ���������ȡ�����һ�ּ�ǿ�������Ե��ʡ�����ݵ������ӿ��γ�һ�ֵ���ɫ����B���ù����ˮ��Ӧ�ɵõ��۵ĵ��ʡ���B����ʽ ���ҵĽṹʽ �������Ԫ�ص�ԭ�ӽṹʾ��ͼ ����֤�������Ԫ�طǽ����Ժ�ǿ����ʵ ��(�ξ�һ������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ��һ��ѧ����ĩ��ѧģ���Ծ� ���ͣ������
(16�֣�����ΪԪ�����ڱ��е�һ���֣���ע��������������ѧ�����ش��������⣺
|
��A |
��A |
��A |
��A |
��A |
��A |
��A |
0 |
|
|
2 |
|
|
|
|
|
�� |
�� |
|
|
3 |
�� |
�� |
�� |
|
|
|
|
�� |
|
4 |
�� |
�� |
|
|
|
|
|
|
�� 8��Ԫ���У���ѧ��������õ��� ���ǽ�������ǿ���� ��
�� �٢ڢݵ�����������Ӧˮ����ļ�����ǿ������˳��Ϊ ��
�� �ڢۢ����γɵļ����Ӱ뾶�ɴ�С��˳��Ϊ ��
�� ���⻯�ﳣ������Ԫ�آڵĹ������ﷴӦ�����ӷ���ʽΪ ��
�� �ٺ͢��γɵĵ���ɫ����ĵ���ʽΪ ��������ѧ��������Ϊ������Ӽ������ۼ����� ��
�� �ٺ͢ݵ�����������Ӧˮ�������Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com