
Ϊ�ⶨδ֪Ũ�ȵ�������Һ��ʵ�����£���1.00mL������������100 mLϡH2SO4��Һ����0.14mol
��L��NaOH��Һ�ζ�����ϡH2SO425.00mL���ζ���ֹʱ����NaOH��Һ15.00mL��
��1����ѧ���ñ�0.14 mol��L NaOH��Һ�ζ������ʵ��������£�
| A������ʽ�ζ���ȡϡH2SO4 25.00 mL��ע����ƿ�У�����ָʾ���� |
| B���ô��ⶨ����Һ��ϴ��ʽ�ζ��ܡ� |
| C��������ˮϴ�ɾ��ζ��ܡ� |
| D��ȡ�¼�ʽ�ζ����ñ���NaOH��Һ��ϴ����Һע���ʽ�ζ��̶ܿȡ�0������2��3 cm������ |
��1����ECDBAGF �ڷ�̪ �۵������һ��NaOH��Һ����ҺͻȻ��ɺ�ɫ������Ӳ���ɫ
��2��ƫ�� �ζ����ڱ��ϵ�ˮĤ������Һϡ�ͣ�ʹ�������ƫ��
���������������1���ٸ��ݵζ�ԭ����ʵ��Ҫ���֪���ζ���������ȷ˳����ECDBAGF��
���������������ǡ�÷�Ӧʱ��Һ�������ԣ��������ѡ���ָʾ���Ƿ�̪��
�۷�̪��ָʾ��������ȷ���յ�ķ����ǵ������һ��NaOH��Һ����ҺͻȻ��ɺ�ɫ������Ӳ���ɫ��
��2�����ڵζ����ڱ��ϵ�ˮĤ������Һϡ�ͣ�ʹ�������ƫ�����Լ�ʽ�ζ���������ˮ��ϴ��
δ�ñ�Һ��ϴ���ᵼ�µζ����ƫ��
���㣺�����к͵ζ�������ָʾ�������ζ��յ���жϺ��й�������
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ��������ѧ��������֪ʶ�Թ���
��Ч�ʣ�����������ѧ���淶���Ͻ���ʵ����ơ��������������������ѧ����Ӧ��������ѧ��������
������ѵ���������������Ҫ��ȷ������������������Ϊ����C��==C��V��/V�� ����C�ꡢV����Ϊ
��ֲ������C��Ĵ�Сȡ����V��Ĵ�С����V�꣺ƫ���ƫС����C��ƫ���ƫС��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
ijѧ��Ϊ�ⶨδ֪Ũ�ȵ�������Һ��ʵ�����£���1.00mL������������100mLϡH2SO4��Һ����0.14mol��L-1��NaOH��Һ�ζ�����ϡH2SO4 25.00mL(��̪��ָʾ��)���ζ���ֹʱ����NaOH��Һ15.00mL��
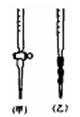
��1����ѧ���ñ�0.14mol��L-1 NaOH��Һ�ζ������ʵ��������£�
A������ʽ�ζ���ȡϡH2SO4 25.00mL��ע����ƿ�У�����ָʾ����
B���ô��ⶨ����Һ��ϴ��ʽ�ζ��ܡ���
C��������ˮϴ�ɾ��ζ���
D��ȡ�¼�ʽ�ζ����ñ���NaOH��Һ��ϴ����Һע���ʽ�ζ��̶ܿȡ�0��
����2��3cm�����ٰѼ�ʽ�ζ��̶ܹ��ã�����Һ�����̶ȡ�0����0���̶�����
E�����ζ����Ƿ�©ˮ������
F����ȡ��ƿ�����ظ�����һ��
G������ƿ���ڵζ������棬ƿ�µ�һ�Ű�ֽ���ߵα�ҡ����ƿֱ���ζ��յ㣬���µζ���Һ�����ڿ̶�
�ٵζ���������ȷ˳����(�������д)��E��C��D�� �� �� ��F��
����G���������ȷ���յ㣿 ��
��2����ʽ�ζ���������ˮ��ϴ��δ�ñ�Һ��ϴ���µζ����
���ƫС������ƫ���䡱����
��3������1mol/L��0.1mol/L��NaOH��Һ��Ӧ��0.1mol/L��NaOH��Һ��ԭ����______________ _____ ___��
��4���ñ�NaOH��Һ�ζ�ʱ��Ӧ����NaOH��Һע�� __��ѡ��ס����ҡ����С�
��5���۲��ʽ�ζ��ܶ���ʱ�����ζ�ǰ���ӣ��ζ����ӣ������ᵼ�²�õ�ϡH2SO4��ҺŨ�� ��ѡ�ƫ��ƫС������Ӱ�족��
��6�������������(ϡ��ǰ������)�����ʵ���Ũ��(������С������λ) ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
Ϊ�ⶨδ֪Ũ�ȵ�������Һ��ʵ�����£���1.00mL������������100 mLϡH2SO4��Һ����0.14mol
��L��NaOH��Һ�ζ�����ϡH2SO425.00mL���ζ���ֹʱ����NaOH��Һ15.00mL��
��1����ѧ���ñ�0.14 mol��L NaOH��Һ�ζ������ʵ��������£�
A������ʽ�ζ���ȡϡH2SO4 25.00 mL��ע����ƿ�У�����ָʾ����
B���ô��ⶨ����Һ��ϴ��ʽ�ζ��ܡ�
C��������ˮϴ�ɾ��ζ��ܡ�
D��ȡ�¼�ʽ�ζ����ñ���NaOH��Һ��ϴ����Һע���ʽ�ζ��̶ܿȡ�0������2��3 cm������
�Ѽ�ʽ�ζ��̶ܹ��ã�����Һ�����̶ȡ�0����0���̶����¡�
E�����ζ����Ƿ�©ˮ��
F����ȡ��ƿ�����ظ�����һ�Ρ�
G������ƿ���ڵζ������棬ƿ�µ�һ�Ű�ֽ���ߵα�ҡ����ƿֱ���ζ��յ㣬���µζ���Һ�����ڿ̶ȡ�
�ٵζ���������ȷ˳����(�������д)����������������������������������
�ڸõζ�������Ӧѡ�õ�ָʾ���ǣ� ����������������������������������
����G���������ȷ���յ�? ��
��2����ʽ�ζ���������ˮ��ϴ��δ�ñ�Һ��ϴ���µζ����(�ƫС������ƫ��ǡ�ú��ʡ�) ��ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ�߶��ڶ��Σ�12�£��¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
��9�֣�ijѧ��Ϊ�ⶨδ֪Ũ�ȵ�������Һ��ʵ�����£���1.00mL������������100 mLϡH2SO4��Һ����0.14mol��L��NaOH��Һ�ζ�����ϡH2SO425.00mL���ζ���ֹʱ����NaOH��Һ15.00mL��
(1)��ѧ���ñ�0.14 mol��L NaOH��Һ�ζ������ʵ��������£�
A������ʽ�ζ���ȡϡH2SO4 25.00 mL��ע����ƿ�У�����ָʾ����
B���ô��ⶨ����Һ��ϴ��ʽ�ζ��ܡ�
C��������ˮϴ�ɾ��ζ��ܡ�
D��ȡ�¼�ʽ�ζ����ñ���NaOH��Һ��ϴ����Һע���ʽ�ζ��̶ܿȡ�0������2��3 cm�����ٰѼ�ʽ�ζ��̶ܹ��ã�����Һ�����̶ȡ�0����0���̶����¡� E�����ζ����Ƿ�©ˮ�� F����ȡ��ƿ�����ظ�����һ�Ρ� G������ƿ���ڵζ������棬ƿ�µ�һ�Ű�ֽ���ߵα�ҡ����ƿֱ���ζ��յ㣬���µζ���Һ�����ڿ̶ȡ�
a���ζ���������ȷ˳����(�������д)�� �� ��
b���õζ�������Ӧѡ�õ�ָʾ���ǣ� �� ��
c����G���������ȷ���յ�? �� ��
(2)��ʽ�ζ���������ˮ��ϴ��δ�ñ�Һ��ϴ���µζ����(�ƫС������ƫ��ǡ�ú��ʡ�) �� ��ԭ���� �� ��
(3)�����������(ϡ��ǰ������)��Һ�����ʵ���Ũ��(��������ȷ��С������λ)�� mol/L
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com