
 ЈЁ1Ј©°СОпЦКЅшРР·ЦАаЈ¬КЗИПК¶ОпЦКµДЧйіЙЎўЅб№№ЎўРФЦКєНУГНѕµД±гЅЭНѕѕ¶Ј®ЗлУГ·ЦАаµД·Ѕ·ЁАґИПК¶ТФПВОпЦКЈЁМоРтєЕЈ©ЈєўЩNaClѕ§Ме ўЪЅрКфН ўЫСОЛб ўЬSO2 ўЭХбМЗ
ЈЁ1Ј©°СОпЦКЅшРР·ЦАаЈ¬КЗИПК¶ОпЦКµДЧйіЙЎўЅб№№ЎўРФЦКєНУГНѕµД±гЅЭНѕѕ¶Ј®ЗлУГ·ЦАаµД·Ѕ·ЁАґИПК¶ТФПВОпЦКЈЁМоРтєЕЈ©ЈєўЩNaClѕ§Ме ўЪЅрКфН ўЫСОЛб ўЬSO2 ўЭХбМЗ
| ||
| ||
| ||
| ||


 ЧЦґКѕд¶ОЖЄПµБРґр°ё
ЧЦґКѕд¶ОЖЄПµБРґр°ё
| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЈЁ10·ЦЈ©ЈЁ1Ј©°СОпЦКЅшРР·ЦАа, КЗИПК¶ОпЦКµДЧйіЙЎўЅб№№ЎўРФЦКєНУГНѕµД±гЅЭНѕѕ¶ЎЈЗлУГ·ЦАаµД·Ѕ·ЁАґИПК¶ТФПВОпЦКЈЁМоРтєЕЈ©Јє
ўЩNaClѕ§Ме ўЪЅрКфН ўЫСОЛб ўЬSO2ўЭХбМЗ ўЮ BaSO4ўЯґїґЧЛб
I ДЬµјµзµДКЗ____________Ј»
IIТФЙПОпЦККфУЪµзЅвЦКµДКЗ____________Ј»
III КфУЪ·ЗµзЅвЦКµДКЗ____________Ј»
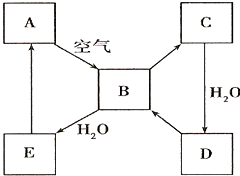
ЈЁ2Ј©ПВНј±нКѕДіµ»ЖЙ«№ММ¬µҐЦКAј°Жд»ЇєПОпЦ®јдµДЧЄ»Ї№ШПµЈЁДіР©ІъОпєН·ґУ¦МхјюТСВФИҐЈ©ЎЈBєНCµДПа¶Ф·ЦЧУЦКБїПаІо16Ј¬»ЇєПОпDКЗЦШТЄµД№¤ТµФБПЎЈ
I µҐЦКAµДГыіЖ ЎЈ
II .РґіцDµДЕЁИЬТєУлCuјУИИ·ґУ¦ЙъіЙBµД»ЇС§·ЅіМКЅ________________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2011-2012С§ДкЙЅ¶«јГДПЖЅТхТ»ЦРёЯТ»ЙПС§ЖЪЖЪД©јмІв»ЇС§КФѕнЈЁґшЅвОцЈ© МвРНЈєМоїХМв
ЈЁ10·ЦЈ©ЈЁ1Ј©°СОпЦКЅшРР·ЦАа, КЗИПК¶ОпЦКµДЧйіЙЎўЅб№№ЎўРФЦКєНУГНѕµД±гЅЭНѕѕ¶ЎЈЗлУГ·ЦАаµД·Ѕ·ЁАґИПК¶ТФПВОпЦКЈЁМоРтєЕЈ©Јє
ўЩNaClѕ§Ме ўЪЅрКфН ўЫСОЛб ўЬSO2 ўЭХбМЗўЮ BaSO4ўЯґїґЧЛб
I ДЬµјµзµДКЗ____________Ј»
IIТФЙПОпЦККфУЪµзЅвЦКµДКЗ____________Ј»
III КфУЪ·ЗµзЅвЦКµДКЗ____________Ј»
ЈЁ2Ј©ПВНј±нКѕДіµ»ЖЙ«№ММ¬µҐЦКAј°Жд»ЇєПОпЦ®јдµДЧЄ»Ї№ШПµЈЁДіР©ІъОпєН·ґУ¦МхјюТСВФИҐЈ©ЎЈBєНCµДПа¶Ф·ЦЧУЦКБїПаІо16Ј¬»ЇєПОпDКЗЦШТЄµД№¤ТµФБПЎЈ
I µҐЦКAµДГыіЖ ЎЈ
II .РґіцDµДЕЁИЬТєУлCuјУИИ·ґУ¦ЙъіЙBµД»ЇС§·ЅіМКЅ________________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2010-2011С§ДкЙЅОчКЎёЯИэЙПС§ЖЪЖЪЦРїјКФ»ЇС§КФѕн МвРНЈєМоїХМв
ЈЁ10·ЦЈ©ЈЁ1Ј©°СОпЦКЅшРР·ЦАа, КЗИПК¶ОпЦКµДЧйіЙЎўЅб№№ЎўРФЦКєНУГНѕµД±гЅЭНѕѕ¶ЎЈЗлУГ·ЦАаµД·Ѕ·ЁАґИПК¶ТФПВОпЦКЈЁМоРтєЕЈ©Јє
ўЩNaClѕ§Ме ўЪЅрКфН ўЫСОЛб ўЬSO2 ўЭХбМЗ ўЮ BaSO4ўЯґїґЧЛб
I ДЬµјµзµДКЗ____________Ј»
IIТФЙПОпЦККфУЪµзЅвЦКµДКЗ____________Ј»
III КфУЪ·ЗµзЅвЦКµДКЗ____________Ј»
ЈЁ2Ј©ПВНј±нКѕДіµ»ЖЙ«№ММ¬µҐЦКAј°Жд»ЇєПОпЦ®јдµДЧЄ»Ї№ШПµЈЁДіР©ІъОпєН·ґУ¦МхјюТСВФИҐЈ©ЎЈBєНCµДПа¶Ф·ЦЧУЦКБїПаІо16Ј¬»ЇєПОпDКЗЦШТЄµД№¤ТµФБПЎЈ
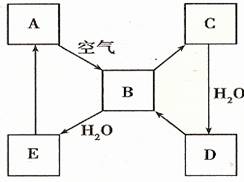
I µҐЦКAµДГыіЖ ЎЈ
II .РґіцDµДЕЁИЬТєУлCuјУИИ·ґУ¦ЙъіЙBµД»ЇС§·ЅіМКЅ________________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє09-10ДкёЈЦЭ°ЛЦРёЯТ»ПВС§ЖЪЖЪЦРїјКФ»ЇС§ѕн МвРНЈєМоїХМв
ЈЁ10·ЦЈ©ЈЁ1Ј©°СОпЦКЅшРР·ЦАа, КЗИПК¶ОпЦКµДЧйіЙЎўЅб№№ЎўРФЦКєНУГНѕµД±гЅЭНѕѕ¶ЎЈЗлУГ·ЦАаµД·Ѕ·ЁАґИПК¶ТФПВОпЦКЈЁМоРтєЕЈ©Јє
ўЩNaClѕ§Ме ўЪЅрКфН ўЫСОЛб ўЬSO2 ўЭХбМЗ ўЮ BaSO4ўЯґїґЧЛб
I ДЬµјµзµДКЗ____________Ј»
IIТФЙПОпЦККфУЪµзЅвЦКµДКЗ____________Ј»
III КфУЪ·ЗµзЅвЦКµДКЗ____________Ј»
ЈЁ2Ј©ПВНј±нКѕДіµ»ЖЙ«№ММ¬µҐЦКAј°Жд»ЇєПОпЦ®јдµДЧЄ»Ї№ШПµЈЁДіР©ІъОпєН·ґУ¦МхјюТСВФИҐЈ©ЎЈBєНCµДПа¶Ф·ЦЧУЦКБїПаІо16Ј¬»ЇєПОпDКЗЦШТЄµД№¤ТµФБПЎЈ
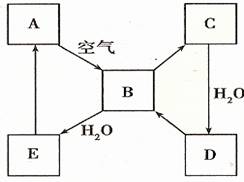
I µҐЦКAµДГыіЖ ЎЈ
II .РґіцDµДЕЁИЬТєУлCuјУИИ·ґУ¦ЙъіЙBµД»ЇС§·ЅіМКЅ________________ЎЈ
Ійїґґр°ёєНЅвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com