
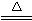 ?
?
ͼ15-39
��֪��2C![]() H
H![]() +(4x+y)CuO
+(4x+y)CuO![]() 2xCO2+(4x+y)Cu+yH2O?
2xCO2+(4x+y)Cu+yH2O?
(1)��ֻ�ⶨSO2���壬����ѡ�õ�װ���ǣ� ����װ����ţ���ͬ������ֻ�ⶨ��������е�CO2������ѡ�õ�װ���� ��?
��2������������ֱ�����ͨ���ۢܢ٢ڢݺۢ͢ܢݢڢ�,�Ƿɲ������������������������ ����ܡ����ܡ�����?
��3�����������������ΪM g���ֱ�����ͨ���ۢܢ٢ڢݣ�ʵ���װ�ü���M1 g����װ������M2 g����ԭ���������CH4����������w (CH4) ��?
��4��������������м��黻����ϩ���������䣨��֪��3CH2![]() CH2+2KMnO4(ϡ��+4H2O
CH2+2KMnO4(ϡ��+4H2O![]() 3CH2OH��CH2OH+2KOH+2MnO2��)����ÿ��ԭ�������ȡ����ΪM g��ֻѡ��װ���еĢۢܢݣ��ܷ�ⶨ���������SO2������������ ����ܡ����ܡ���������ܣ����Ҫд�������Ʒ����ͽ����?��������������?����������������������������??
3CH2OH��CH2OH+2KOH+2MnO2��)����ÿ��ԭ�������ȡ����ΪM g��ֻѡ��װ���еĢۢܢݣ��ܷ�ⶨ���������SO2������������ ����ܡ����ܡ���������ܣ����Ҫд�������Ʒ����ͽ����?��������������?����������������������������??
��1���ۡ��ۢ٢ܻ�ۢ٢ݣ���ܻۢ�ۢ�Ҳ�ɣ�?
��2���ܡ���3��![]() ?
?
(4)�ܡ���ȡm g�����������ͨ��װ�âۣ����װ�â����ӵ���������Ϊa g����ȡm g���������������ͨ��װ�â���ͨ��װ�âۣ���������ӵ�����b g��w(SO2)=![]() ��
��
��������1��SO2��CO2�����������壬�Կɱ������ա���SO2���н�ǿ��ԭ�ԣ�CO2���������ԣ���ֻ�ⶨSO2ʱ����ѡ��װ�âۡ���ֻ�ⶨCO2��������SO2���پ�����ܱ������գ�����ѡ�õ�װ��Ϊ�ۢ٢ܻ�ۢ٢ݡ���2�����徭���ۿɲ��SO2��������ͨ���ܿɲ��CO2�����������ٸ������ͨ���ڣ���ʱ����H2��CH4�����ʵ�����ʾ��Ӧǰ��������������â����ص����ֿɵõ���H2��CH4���ʵ����ķ��̣�����ö��ߵ����ʵ������������������һ�£�˳��ߵ�����Ӱ������������˳���������Ҫ��?
��3����CH4��H2�����ʵ����ֱ�Ϊn(CH4)��n(H2),��?
CH4+4CuO![]() 4Cu+CO2��+��2H2O����������?
4Cu+CO2��+��2H2O����������?
1 mol 1 mol 2 mol
n(CH4) n(CH4) 2n(CH4) 64n(CH4)����
H2+CuO![]() Cu+H2O����������?
Cu+H2O����������?
1 mol 1 mol
n(H2) n(H2) 16n(H2)?
 ?
?
(4)�ܵ�������Ȼ�DzⶨCO2����������ʹ�������ͨ��װ�âۣ���������������Ϊ��ϩ��SO2�������͡���ȡ����������ͨ��װ�âݣ���SO2�����գ���ͨ����ʱ������Ϊ��ϩ�������������ΪSO2����������������SO2������������


 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(15��)��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݡ�
��.�������A-D��ʾ�����ʣ����¹�����Ҫ������Ũ�������Щ���ʣ��뽫ѡ����ĸ�������и�С��������ڣ�
Aǿ���� B ��ˮ�� C ��ˮ�� D ǿ������
��1��Ũ������Ը��������� ��
��2��Ũ����ʹľ����ڣ� ��
��3���ȵ�Ũ������ͭƬ��Ӧ�� ��
�����ø�Ũ��������100 mL��1 mol/L��ϡ���ᡣ�ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ�����ƿ�����ձ����� ҩ�ף�����Ͳ����������ƽ��
��ش��������⣺
(1)����ϡ����ʱ�����������в���Ҫʹ�õ��� ��ѡ����ţ�����ȱ�ٵ�������
���� ��д�������ƣ���
(2)�����㣬����100mL1mol/L��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ �� mL������һλС��������ȡŨ����ʱӦѡ�� ��ѡ���10mL����50mL ����100mL��������Ͳ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�γ���������ѧ������ѧ��ѧ����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(15 ��) S2Cl2�ǹ�ҵ�ϳ��õ�����ʵ�����Ʊ�S2Cl2�ķ�Ӧԭ�������֣�
�� CS2��3Cl2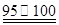 ��CCl4��S2Cl2���� 2S��Cl2
��CCl4��S2Cl2���� 2S��Cl2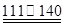 ��S2Cl2��
��S2Cl2��
��֪��S2Cl2����ˮ��Ӧ��S2Cl2��Cl2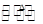 2SCl2��
2SCl2��
�����Ǽ������ʵ��۷е�ͷ�Ӧװ��ͼ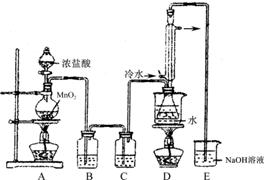
| ���� | �е�/�� | �۵�/�� |
| S | 445 | 113 |
| CS2 | 47 | ��109 |
| CCl4 | 77 | ��23 |
| S2Cl2 | 137 | ��77 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�γ��и�����ѧ��ѧ����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
(15 ��) S2Cl2�ǹ�ҵ�ϳ��õ�����ʵ�����Ʊ�S2Cl2�ķ�Ӧԭ�������֣�
�� CS2��3Cl2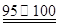 ��CCl4��S2Cl2���� 2S��Cl2
��CCl4��S2Cl2���� 2S��Cl2 ��S2Cl2��
��S2Cl2��
��֪��S2Cl2����ˮ��Ӧ��S2Cl2��Cl2 2SCl2��
2SCl2��
�����Ǽ������ʵ��۷е�ͷ�Ӧװ��ͼ

|
���� |
�е�/�� |
�۵�/�� |
|
S |
445 |
113 |
|
CS2 |
47 |
��109 |
|
CCl4 |
77 |
��23 |
|
S2Cl2 |
137 |
��77 |
��1��������ͼװ�ã����ּг���������ȥ�����Ʊ�S2Cl2����Ӧԭ���� ����д�������ַ�Ӧԭ����������ţ�
��2����װ��C�����ɸ���ܣ���װ��C�п�ѡ�õĹ����Լ��� ��
��3��Dװ���������ܵ������� ����Ӧ������Dװ����ƿ�ڵĻ�����з��������ķ����� ��
��4��S2Cl2������ˮ��Ӧ�л�ɫ�������ɣ���������ɫ������ʹƷ����Һ��ɫ����÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��Ϊ������ƵõIJ�ƷS2Cl2�Ĵ��ȣ��ؼ��IJ����ǿ��ƺ��¶Ⱥ� ��
��6����ͼβ��װ�ò������ƣ����ڵ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
(15��)��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݡ�

��.�������A-D��ʾ�����ʣ����¹�����Ҫ������Ũ�������Щ���ʣ��뽫ѡ����ĸ�������и�С��������ڣ�
Aǿ���� B ��ˮ�� C ��ˮ�� D ǿ������
��1��Ũ������Ը��������� ��
��2��Ũ����ʹľ����ڣ� ��
��3���ȵ�Ũ������ͭƬ��Ӧ�� ��
�����ø�Ũ��������100 mL��1 mol/L��ϡ���ᡣ�ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ�����ƿ�����ձ����� ҩ�ף�����Ͳ����������ƽ��
��ش��������⣺
(1)����ϡ����ʱ�����������в���Ҫʹ�õ��� ��ѡ����ţ�����ȱ�ٵ�������
���� ��д�������ƣ���
(2)�����㣬����100mL1mol/L��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ �� mL������һλС��������ȡŨ����ʱӦѡ�� ��ѡ���10mL����50mL ����100mL��������Ͳ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com