
(16��)���仯����������ת����ϵ
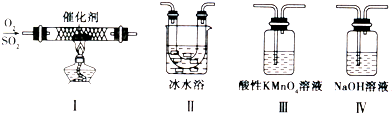
(1)����������ԭ��Ӧ���� (�����)
(2)д��(5)�ķ�Ӧ����ʽ ��
˵��Ũ������� ����ȡCuSO4�� �������(�����)��
(3)SO2����ɿ�����Ⱦ���γ��������Ҫ���ʡ�SO2��ˮ�Ĵ�������������Ӧ�������ᣬ��Ӧ����ʽΪ ��
��֤��������ķ����� ��
(4)ij��Һ�к���Cl-��SO42-�����ܺ���Na+��Fe2+������һ�֡�
����֤Cl-��SO42-�ķ�����
A.�ȼ�BaCl2��Һ���ȳ������ټ�AgNO3��Һ
B.�ȼ�AgNO3��Һ���ȳ������ټ�BaCl2��Һ
C.�ȼ�Ba(NO3)2��Һ���ȳ������ټ�AgNO3��Һ
����֤Na+��Fe2+��ķ����� ��
(16��)
(1) (1)(2)(5) (2��)
(2)2H2SO4(Ũ)+Cu CuSO4+SO2��+2H2O�� (2��)
CuSO4+SO2��+2H2O�� (2��)
ǿ�������� (6) (��1��=2��)
(3) 2SO2+O2+2H2O===2H2SO4 (2��)
��ij����ͨ��ʢƷ����Һ���Թ�����Ʒ����ɫ���ټ������ֳ��ֺ�ɫ��֤����SO2�� (3��)
(4)�� C (2��)
������ҺΪ��ɫ������Һ��ֻ��Na+������Һ����ɫ��һ����Fe2+��������ɫ��Ӧ����֤��Na+�Ĵ��ڡ�(����������Ҳ����) (3��)
����


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��1�� | ��2�� | ��3�� |
| S�����ʣ� | SO2��H2SO3��M��NaHSO3 | SO3��H2SO4��Na2SO4��NaHSO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(16��) ���仯����������ת����ϵ
(1)����������ԭ��Ӧ���� (�����)
(2)д��(5)�ķ�Ӧ����ʽ ��
˵��Ũ������� ����ȡCuSO4�� �������(�����)��
(3)SO2����ɿ�����Ⱦ���γ��������Ҫ���ʡ�SO2��ˮ�Ĵ�������������Ӧ�������ᣬ��Ӧ����ʽΪ ��
��֤��������ķ����� ��
(4)ij��Һ�к���Cl-��SO42-�����ܺ���Na+��Fe2+������һ�֡�
����֤Cl-��SO42-�ķ�����
A.�ȼ�BaCl2��Һ���ȳ������ټ�AgNO3��Һ
B.�ȼ�AgNO3��Һ���ȳ������ټ�BaCl2��Һ
C.�ȼ�Ba(NO3)2��Һ���ȳ������ټ�AgNO3��Һ
����֤Na+��Fe2+��ķ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�����ڶ��ζο������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
(16��)�����仯����������������й㷺Ӧ�á���ش��������⣺
��1��������FeS2�������������ұ����������Ҫԭ�ϡ�����һ����ӦΪ
3FeS2��8O2 ==6SO2��Fe3O4����������Ϊ ������3 mol FeS2�μӷ�Ӧ��
==6SO2��Fe3O4����������Ϊ ������3 mol FeS2�μӷ�Ӧ��
ת�� mol���ӡ�
��2���Ȼ�����Һ������ӡˢ��·ͭ�帯ʴ������Ӧ�����ӷ���ʽΪ ��
 ��3�����������ƣ�������Ҳ��������ˮ������ԭ��Ϊ
�������ӷ��̱�ʾ��
��3�����������ƣ�������Ҳ��������ˮ������ԭ��Ϊ
�������ӷ��̱�ʾ��
��4���ٸ����ĵ绯��ʴ��ʾ��ͼ���ң�����ͼ������ ���ɳ�Ϊ�����绯ѧ�����ļ�ʾ��ͼ��������ͼ���߿��������ģ����ü�ͷ���������������
��д����ǰ�ĸ���������ʴʯī�缫�ĵ缫��Ӧʽ ��
��5��������һ�ֺ�ɫ���ϣ���ɷ���Fe2O.3��һ��������������160ml5 mol��L��1�����У��ڼ���һ��������ǡ���ܽ⣬�ռ���2.24L����״����������⣬��ҹ����Fe3+����μӷ�Ӧ�����۵�����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ��һ��ѧ����ĩ���⻯ѧ�������Ծ� ���ͣ������
(16��) ���仯����������ת����ϵ
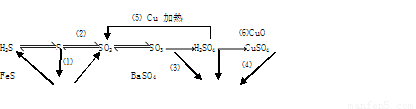
(1)����������ԭ��Ӧ���� (�����)
(2)д��(5)�ķ�Ӧ����ʽ ��
˵��Ũ������� ����ȡCuSO4�� �������(�����)��
(3)SO2����ɿ�����Ⱦ���γ��������Ҫ���ʡ�SO2��ˮ�Ĵ�������������Ӧ�������ᣬ��Ӧ����ʽΪ ��
��֤��������ķ����� ��
(4)ij��Һ�к���Cl-��SO42-�����ܺ���Na+��Fe2+������һ�֡�
����֤Cl-��SO42-�ķ�����
A.�ȼ�BaCl2��Һ���ȳ������ټ�AgNO3��Һ
B.�ȼ�AgNO3��Һ���ȳ������ټ�BaCl2��Һ
C.�ȼ�Ba(NO3)2��Һ���ȳ������ټ�AgNO3��Һ
����֤Na+��Fe2+��ķ����� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com