

���� ��1�����ʳ��ӵ�ԭ���ǡ��������������֡���ԭ����ע��һ�㣬�Ǿ�����������������Na2CO3��Һ��Ϊ�˳�ȥ������Ca2+�Ͷ����Ba2+��Ȼ����й��ˣ���ȥ���ɵ������Ȼ��������������ᣬ��ȥ�����CO32-�͵�����Һ������ԣ������������ᾧ�õ�������NaCl���壻����̼��Ƶ��ܶȻ��������㣻
��2�������ȡNaOH�����ӷ�Ӧ�ǵ�ⱥ��ʳ��ˮ�ķ�Ӧ���������������ӵõ������ƻ���ˮ�ĵ���ƽ�⣬����������Ũ������
��3��ʯ��ʯ�������ɵ�����MΪCO2�����������պ�IJ�����Ҫ��SiO2��Al2O3����NaOH���˺�õ�����ҺN��Ҫ����NaAlO2��Na2SiO3���Դ���д���ӷ���ʽ��
��4��̼����ˮ��ʼ��ԣ�����CO32-���Ӵ�������ˮ�⣬�ҵ�һ��ˮ����ڵڶ���ˮ�⣬�Դ��ж�����Ũ�ȴ�С˳��
��4��ʯ��ʯ�ֽ����ɶ�����̼���壬���ڹ�ҵ���ɣ����ɵ���ҺN�к���̼�����ƣ����Ⱥ������ɶ�����̼���壬��ѭ�����ã�
��5�������������������غ���㣮
��� �⣺��1�����ʳ��ӵ�ԭ���ǡ��������������֡���ԭ���������˽���������Ŀ�ģ����ܷ������˳���������NaOH��Һ��Ϊ�˳�ȥMg2+�����������BaCl2��Һ��Ϊ�˳�ȥSO42-�����������Na2CO3��Һ��Ϊ�˳�ȥ������Ca2+�Ͷ����Ba2+��Ȼ����й��ˣ���ȥ���ɵ������Ȼ��������������ᣬ��ȥ�����CO32-�͵�����Һ������ԣ������������ᾧ�õ�������NaCl���壮���������˳���Ҫ����Na2CO3��Һ������BaCl2��Һ֮����룬���������ᣬ����̼��Ƶ��ܶȻ��������㣬ҪʹCa2+��ȫ������c��Ca2+����10-5mol/LӦ��
c��Ca2+����c��CO2-3����2.9��10-9����c��CO2-3����$\frac{2.9��1{0}^{-9}}{1��1{0}^{-5}}$=2.9��10-4mol/L��
�ʴ�Ϊ���ܢ٢ڢۻ�٢ܢڢۻ�ܢڢ٢ۣ�2.9��10-4mol/L��
��2�������ȡNaOH�����ӷ�Ӧ�ǵ�ⱥ��ʳ��ˮ�ķ�Ӧ����Ӧ�����ӷ���ʽΪ��2Cl-+2H2O $\frac{\underline{\;ͨ��\;}}{\;}$ 2OH-+H2��+Cl2�����������������ӵõ������ƻ���ˮ�ĵ���ƽ�⣬����������Ũ��������ҺPH����
�ʴ�Ϊ��2Cl-+2H2O $\frac{\underline{\;ͨ��\;}}{\;}$ 2OH-+H2��+Cl2��������
��3��ʯ��ʯ�������ɵ�����MΪCO2�����������պ�IJ�����Ҫ��SiO2��Al2O3����NaOH���˺�õ�����ҺN��Ҫ����NaAlO2��Na2SiO3������ͨ������CO2������Ӧ�ķ���ʽΪ��NaAlO2+CO2+2H2O=Al��OH��3��+NaHCO3��Na2SiO3+2CO2+2H2O=H2SiO3+2NaHCO3�����ӷ���ʽΪ��SiO32-+2CO2+2H2O�TH2SiO3��+2HCO3-��AlO2-+2H2O+CO2�TAl��OH��3��+HCO3-��
�ʴ�Ϊ��SiO32-+2CO2+2H2O�TH2SiO3��+2HCO3-��AlO2-+2H2O+CO2�TAl��OH��3��+HCO3-��
��4��̼����ˮ��ʼ��ԣ�����CO32-���Ӵ�������ˮ�⣬�ҵ�һ��ˮ����ڵڶ���ˮ�⣬���У�c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
�ʴ�Ϊ��c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
��4��ʯ��ʯ�ֽ����ɶ�����̼���壬���ڹ�ҵ���ɣ����ɵ���ҺN�к���̼�����ƣ����Ⱥ������ɶ�����̼���壬��ѭ�����ã��ʴ�Ϊ��CO2��
��5�������������������غ���㣬�����ɷ���ɸ������Ϊx������1t��35%=28%��xt��x=$\frac{35}{28}$t=1.25t��
�ʴ�Ϊ��1.25��
���� ������һ���ԡ�����ɸ�����Ʊ�Ϊ�����Ļ�ѧ���������⣬������ǻ�ѧ����֪ʶ�������������ʵ�ʣ��Խ����ѧʵ��������˼·�������ʣ�ʹ�����龳��ʵ��������ֻ�ѧ��STSE�Ĺ�ϵ������ѧ�Ա�ɫ���ܿ���ѧ�����Ķ����������ϵ��ռ�����������ͬʱ��ѧ���������������������⡢������⡢���ֱ���ȷ����������Ҫ��dz��ߣ���һ���ۺ������⣬��ѧ���Ժ��ѧϰ���������õ�ָ�����ã���Ŀ�Ѷ��еȣ�


 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д� Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڷŵ������£�N2��O2��ֱ�ӻ�������NO | |
| B�� | NO����������ˮ | |
| C�� | ��ʢNO�����ƿ�ǣ���������ƿ���к���ɫ�������� | |
| D�� | NO�Ǻ���ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ɽ��������ú��3•28ˮ�¹ʱ��������ھ��¿���ľм����Ƥ������ˮά��������ľм����Ƥ�Ļ�ѧ�ɷ���Ҫ����ά�� | |
| B�� | �Ҵ������Ͷ��ǿ�������Դ��Ӧ�����ƹ㡰�Ҵ����͡� | |
| C�� | 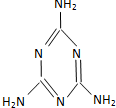 ��Ϊ��֪���̷��¼��е��������Ϊ�����谷���ṹ��ͼ����Ħ������Ϊ126g | |
| D�� | 2010��4��֧Ԯ�ຣ��������������������Ʒ�е�ʳ�ס����ʳ�Σ����Ӧ����Ҫ��ѧ���ʷֱ������ᡢ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ȼ����Ϊ285.8kJ•mol-1��������ȼ�յ��Ȼ�ѧ����ʽΪ��2H2��g��+O2��g���T2H2O��l����H=-285.8 kJ•mol-1 | |
| B�� | ��֪�к���Ϊ57.3 kJ•mol-1������1L1mol•L-1�����뺬1molNaOH��Һ��ϣ��ų�������ҪС��57.3kJ | |
| C�� | Ba��OH��2•8H2O��s��+2NH4Cl��s���TBaCl2��s��+2NH3��g��+10H2O��l����H��0 | |
| D�� | ������������������Ʒֱ���ȫȼ�գ����߷ų��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Na2CO3 | B�� | ����CuSO4 | C�� | �մ�NaHCO3 | D�� | ���NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
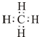 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��
 ��
��
| ��� | ͬλ�� | ͬ�������� | ͬ���칹�� |
| ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ���л���Ļ�ѧʽΪC11H12OCl2 | |
| B�� | ��ͬһƽ���ϵ�̼ԭ�������Ϊ10�� | |
| C�� | ��NaOH����Һ�ڼ�����������ȥ��ԭ�� | |
| D�� | ��ͭ�������ͼ����������ܱ�O2������ȩ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com