
| 13 |
| 2 |
| 13 |
| 2 |


 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2009?�ɶ�һģ��һ���л������Ľṹ��ʽ��ͼ��ʾ�����й���������������ȷ���ǣ�������
��2009?�ɶ�һģ��һ���л������Ľṹ��ʽ��ͼ��ʾ�����й���������������ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
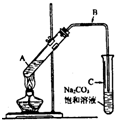 ��2009?�ɶ�һģ��ij��ȤС��������ͼװ�ã�ȡ��ͬŨ�ȵ������� 3mL ��ˮ�Ҵ��� 2mL ������ֱ������ȡ�����������о���
��2009?�ɶ�һģ��ij��ȤС��������ͼװ�ã�ȡ��ͬŨ�ȵ������� 3mL ��ˮ�Ҵ��� 2mL ������ֱ������ȡ�����������о��� CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O| ����� | ���� | ��Ӧ���� | C�б���̼������Һ������߶� |
| �� | 2mL98%Ũ���� | 20��ʱ��Һ������ɫ���淴Ӧ���У���Һ��ɫ������ɺ�ɫ�����������ݣ� | 2.10cm |
| �� | 2mL14mol?L-1���� | ��Ӧ����Һ��ɫ����ɫ�������뱥��̼������Һ���������������ݣ� | 2.14cm |
| �� | 2mL10mol?L-1���� | ��Ӧ����Һ��ɫ����ɫ�������뱥��̼������Һ���������������ݣ� | 2.16cm |
| �� | 2mL7mol?L-1���� | ��Ӧ����Һ��ɫ����ɫ�������뱥��̼������Һ�����ݣ� | 2.00cm |
| �� |
| �� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com