
CO�����ںϳɼ״���һ���¶��£������Ϊ2L���ܱ������м���CO��H2��������Ӧ
CO��g��+2H2��g�� CH3OH��g������ƽ����ø����Ũ�����£�
CH3OH��g������ƽ����ø����Ũ�����£�
| ���� | CO | H2 | CH3OH |
| Ũ��(mol•L��1) | 0.9 | 1.0 | 0.6 |
�ٻ�������ƽ����Է�������__________________________��
����ʽ������ƽ�ⳣ��K=__________________________��
�������������ѹ��Ϊ1L,�������㣬Ԥ����ƽ����c(H2)��ȡֵ��Χ��__________��
��������������䣬�ٳ���0.6molCO��0.4molCH3OH,��ʱv��___v��(�>������<����=��)��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ߴ�MnCO3���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������MnO2Ϊԭ���Ʊ������ߴ�MnCO3�IJ����������£�
�������ϣ���MnCO3������ˮ����ʪʱ�ױ�����������100�濪ʼ�ֽ⡣
��Mn��OH��2��ʼ����ʱpH=7.7��
ʵ����̣�
��. ��ͼ1��ʾװ���Ʊ�MnSO4��Һ��
��. �߽������MnSO4��Һ�м����Թ���NaHCO3��Һ�����ˡ�
��. ϴ�ӳ�����
��. ϴ�Ӻ�ij������¶ȵ���100��������¸���Ƶøߴ�MnCO3���塣
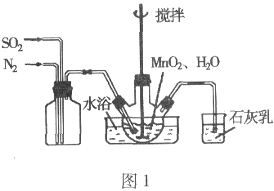
��1��ͼ1��ʯ�����������__________����ƿ�з�����Ӧ�Ļ�ѧ����ʽ��__________��
��2������ͼ2�����̡��ķ�Ӧ����ʱ����____________________����ʵ���н�N2���ɿ�������÷�ӦҺ��Mn2+��SO42-��Ũ���淴Ӧʱ��t�仯��ͼ3��������Һ��Mn2+��SO42-Ũ�ȱ仯�������Բ����ԭ����______________________________��
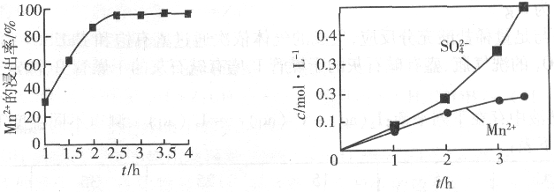
ͼ2 ͼ3
��3�����м����Թ���NaHCO3��Һ��������ҺpH��__________��Χ��д������NaHCO3��������Ӧ�����ӷ���ʽ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ��������������ء�����˵������ȷ���ǣ� ��
A������̫���ܵ������Դ���滯ʯȼ�ϣ������ڽ�Լ��Դ����������
B��������ʳƷ���Ӽ���ʳ������彡�����к�������ʳ��
C��Ϊ��ֹ����е��ؽ�������Ⱦ������ˮ�壬Ӧ���������ϵ�ص��ۺ����ü���
D���ᳫ���ǹ���ʱ�������ϴ�����Ϊ�˷�ֹ��ɫ��Ⱦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����¡����ݣ�2L�����ܱ������г���2molX��һ������Y��������Ӧ��2X(g)��Y(g)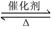 2Z(g)
2Z(g)
��H��0��4min��ﵽƽ��c(X)��0.2mol·L-1����X��Y��ת������ȡ�����˵��������ȷ���ǣ� ��
A���ﵽƽ��ʱ���ٳ���1molX����Ӧ���ʱ䱣�ֲ���
B����Y��ʾ4min�ڵķ�Ӧ����Ϊ0.1 mol·L-1·min-1
C�����������г���1molZ���ﵽ��ƽ�⣬c(X)��c(Y)��2��1
D��4min���������¶ȣ�ƽ�ⳣ��K����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£�0.1mol·L��1ijһԪ��(HA)��Һ��c(OH��)/c(H��)��1��10��8������������ȷ���ǣ� ��
A����pH��3��HA��pH��11��NaOH��Һ�������ϣ���Һ��c(Na��)��c(Ac—)��c(OH��)��c(H��)
B��0.1mol·L��1HA��Һ��0.05mol·L��1NaOH��Һ�������Ϻ�������Һ�У�
2c(H��)��c(HA)��c(A��)��c(OH��)
C������Һ����ˮ�������c(H��)��1��10��11 mol·L��1
D��Ũ�Ⱦ�Ϊ0.1mol/L��HA��NaA��Һ�������Ϻ�����Һ�����ԣ���
c(A��)��c(HA)��c(Na��)��c(H��)��c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ƕ�Ԫ���ᣬ���������Һ�����ԡ���0.1mol·L��1 KHC2O4��Һ�У����й�ϵ��ȷ���� ( )
A��c(K+)+c(H+) = c(HC2O4��)+c(OH��)+c(C2O42��)
B��c(HC2O4��)+c (C2O42��) = 0.1 mol·L��1
C��c(C2O42��) < c(H2C2O4)
D��c(K+) = c(H2C2O4)+c(HC2O4��)+c(C2O42��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��(NH4)2CO3(s)=NH4HCO3(s)+NH3(g) ��H = + 74.9 kJ·mol-1������˵������ȷ���� �� ��
A���÷�Ӧ���ر䡢�ʱ�Դ���0
B���÷�Ӧ�����ȷ�Ӧ�����һ�������Է�����
C��̼���ηֽⷴӦ�������ӣ�����κ�����������̼���ηֽ�һ���Է�����
D�����Է����еķ�Ӧһ���Ƿ��ȷ�Ӧ�������Է����еķ�Ӧһ�������ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ƕ�Ԫ���ᣬ���������Һ�����ԡ���0.1mol·L��1 KHC2O4��Һ�У����й�ϵ��ȷ���� ( )
A��c(K+)+c(H+) = c(HC2O4��)+c(OH��)+c(C2O42��)
B��c(HC2O4��)+c (C2O42��) = 0.1 mol·L��1
C��c(C2O42��) < c(H2C2O4)
D��c(K+) = c(H2C2O4)+c(HC2O4��)+c(C2O42��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݡ����ø�Ũ��������100 mL��1 mol/L��ϡ���ᡣ�ɹ�ѡ�õ������У�
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݡ����ø�Ũ��������100 mL��1 mol/L��ϡ���ᡣ�ɹ�ѡ�õ������У�
�ٽ�ͷ�ιܣ�����ƿ�����ձ����� ҩ�ף�����Ͳ����������ƽ��
��ش��������⣺
(1)����ϡ����ʱ�����������в���Ҫʹ�õ��� ��ѡ����ţ���
��ȱ�ٵ������� ���� ��д�������ƣ���
(2)�����㣬����100mL1mol/L��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ �� mL������һλС��������ȡŨ����ʱӦѡ�� ��ѡ���10mL����50mL ����100mL��������Ͳ��
(3)�����ƹ����У�����ϴ���ձ����õ���Һֱ�ӵ����Һ���У��������������Һ��Ũ��
___ _____ (�ƫ�ߡ�����ƫ�͡����ޱ仯������ͬ)��
�ڶ���ʱ��������ƿ�Ŀ̶��ߣ�������õ���ҺŨ��_____ __��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com