
A��B��C��DΪ������̬���ʣ��ס��ҡ�������������ЩԪ����ɵĻ������֪��
��A��B�����ڷŵ������·������Ϸ�Ӧ�ף�������B�������ɺ���ɫ���壻
��C�ֱ���A��B��Ӧ���ɵ����ֻ������Һͱ��ķ����ж�����10�����ӣ�
��C��D��Ӧ���ɵĻ����ﶡ������ˮ��������Һ�еμ�AgNO3��Һ�����ɰ�ɫ������
������������⣺
(1)C��D��Ӧ���ɵĻ�����ĵ���ʽ��________��
(2)�������е����ֻ��������Ӧ�Ļ�ѧ����ʽΪ��________��
(3)��Dͨ��B��C���ɵĻ������У�������Ӧ�����ӷ���ʽΪ��________��
(4)��ҵ������D����ʱ��Ϊ�����Ƿ����й©�����ù������ҵ�Ũ��ҺͿ�ڹܵ����ϣ���________����ʱ����˵��������D���ʵ�й©����ʱ��Ӧ�Ļ�ѧ����ʽΪ��________��
|
�����𰸣�(1) ����(2)NH3��H2O ����(3)Cl2��H2O ����(4)���̲����ġ�8NH3��3Cl2 ����˼·�����������������֪��AΪN2��BΪO2�������ڷŵ������·�Ӧ���ɵIJ���ΪNO��NO��һ����O2��Ӧ���ɺ���ɫNO2��CΪH2��NH3��H2O��Ϊ10���ӷ��ӣ���D��Cl2��Cl2��NH3��Ӧ�ķ���ʽ��NH3������ʱΪ��2NH3��3Cl2 |


 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ũ���� |
| �� |
| Ũ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���㽭ʡ����ѧ������ѧ��10���¿�����ѧ�� ���ͣ������
��12�֣���֪A��B��C��DΪ�������ʣ�����B��C��D���³�ѹ��Ϊ���壬�ס��ҡ�������Ϊ�����Ļ�����ҳ�����ΪҺ�壬������ɫ��ӦΪ��ɫ����ͼΪ��������֮���ת����ϵ��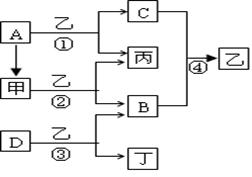
��ش��������⣺
��1��д���������ʵĻ�ѧʽ��
B ��D ��
��2���ĵ���ʽΪ ����Ӧ��������11.2L����״���£�B���ɣ�����ת�Ƶĵ��ӵ����ʵ���Ϊ ��
��3��д����Ӧ�ٵ����ӷ���ʽ�� ��
д����Ӧ�۵Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ����һ��2013�����12���¿���ѧ�Ծ����������� ���ͣ��ƶ���
A��B��C��DΪ�������嵥�ʡ���֪��
��A��B�����ڷŵ������·������Ϸ�Ӧ������ﻹ������B�������ɺ���ɫ���壻
��C�ֱ���A��B��Ӧ���ɵ����ֻ���������ж�����10�����ӡ�
��C��D��Ӧ���ɵĻ�����������ˮ��������Һ�еμ�AgNO3��Һ�����ɰ�ɫ������
��ش�
��1��C��D��Ӧ���ɵĻ�����ĵ���ʽ�� ��
��2���������л���������ȶ��� ǿ�� ������д��ѧʽ��
��3����Dͨ��B��C���ɵĻ������У�������Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com