
ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
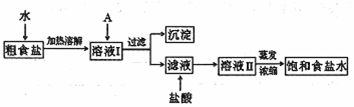
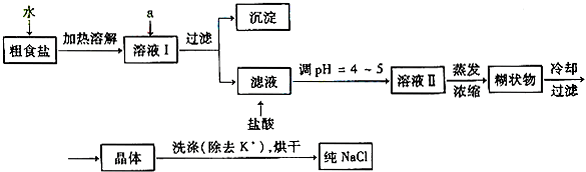
(1)��ʳ�γ���������![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ���������ӣ�ʵ�����ᴿ
���������ӣ�ʵ�����ᴿ![]() ��
��
�� �������£�
���������������������������� 
�ṩ���Լ�������![]() ��Һ�� ����
��Һ�� ����![]() ��Һ��
��Һ�� ![]() ��Һ�� BaCl2��Һ
��Һ�� BaCl2��Һ
�������� �� ![]() ��Һ
��Һ
������ȥ��Һ���е�![]() ��
��![]() ��
��![]() ��
��![]() ���ӣ�ѡ��A���������Լ������μ�˳
���ӣ�ѡ��A���������Լ������μ�˳
������Ϊ���������������������������� (ֻ�ѧʽ)��
�ڼ������Ŀ����(�����ӷ���ʽ��ʾ)�������������������������������� ��
(2)ij��ѧС�����������װ��(�гֺͼ�����������ȥ)��ⱥ��ʳ��ˮ�����õ���
����![]() ��ԭ
��ԭ![]() ��ĩ���ⶨCu�����ԭ��������ͬʱ��֤�����������ԡ�
��ĩ���ⶨCu�����ԭ��������ͬʱ��֤�����������ԡ�
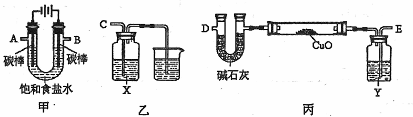
��д�����з�Ӧ�Ļ�ѧ����ʽ������������������������ ������������ ������
��Ϊ�������ʵ�飬��ȷ������˳��ΪA������ ��B������ (��ӿ���ĸ)��
����װ����X�Լ����������������� ����װ����Y�Լ��������������������� ��
�ܲⶨCu�����ԭ��������
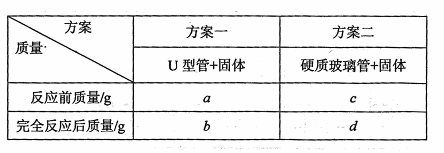
��![]() g
g![]() ����Ӳ�ʲ������У�����������������õ����ݼ���Cu�����ԭ��������
����Ӳ�ʲ������У�����������������õ����ݼ���Cu�����ԭ��������
��
��ش�:����
�� ����Ϊ������������ �ϼѣ���һ�������õ���Cu�����ԭ�������������� ���ƫ
�͡�����ƫ�ߡ�)�����ϼѷ������㣬�õ�Cu�����ԭ������������������������������ ��
 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д� ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��ը����ʱ1 kg��������0.5 kgˮ��4 g������10 gС�մ�����ʳ�εȸ��ϣ�����ը����Ʒ�����IJ���һ��Ϊ80%��ͨ������˵����ÿ��ʳ��100 g��������������������__________________��?
��2�����о��ҹ��������ճ�����������������ʳƷ���Ӽ��⣩�����ֿ���;����______________________________________________________________________?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0115 �¿��� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com