
����TiO2��Ϳ�ϡ��������ױƷ���������ż���㷺��Ӧ�á�
��1����ҵ�϶������ѵ��Ʊ��ǣ� ���Ͽ�Ƭ��
�����۵�SiCl4 ��70 �� �� TiCl4 ��25 �棻���ʷе�SiCl457.6 �桢TiCl4136.5 ��
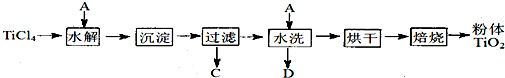
�������Ľ��ʯ����Ҫ�ɷ�TiO2����Ҫ����SiO2����̼�ۻ��װ���Ȼ�¯�У��ڸ�����ͨ��Cl2��Ӧ���Ƶû���SiCl4���ʵ�TiCl4��
��SiCl4���룬�õ�������TiCl4��
����TiCl4�м�ˮ�����ȣ�ˮ��õ�����TiO2��xH2O��
���������ˡ�ˮϴ��ȥ���е�Cl�����ٺ�ɡ����ճ�ȥˮ�ֵõ�����TiO2 ��
���ڳ����·���TiCl4��SiCl4����ȡ�IJ��������� ��
�ڢ��з�Ӧ�Ļ�ѧ����ʽ�� ��
�� ����TiO2��x H2O��Cl���Ƿ����ķ�����______________________________��
��2��TiO2���ӵĴ�С�������ִ����������ⶨ������������ԭ�ζ����ɲⶨTiO2������������һ�������£���TiO2�ܽⲢ��ԭΪTi3+ ������KSCN��Һ��ָʾ������NH4Fe(SO4)2����Һ�ζ�Ti3+��ȫ������Ti4+���ش��������⣺
�����п����ڲⶨTiO2���Ӵ�С�ķ�����_____________________������ĸ���ţ���
a.�˴Ź��� b.������� c.���� d.�����������
������NH4Fe(SO4)2����Һʱ��ʹ�õ���������ƽ��ҩ�ס����������ձ�����Ͳ�⣬����Ҫ��ͼ�е�_____������ĸ���ţ���
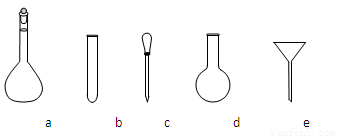
�۵ζ��յ��������___________________________________________________��
�ܵζ�����ʱ����ȡTiO2��Ħ������ΪM g��mol��1������w g������c mol/L NH4Fe(SO4)2����ҺV mL����TiO2������������ʽΪ_________________________��
���������Ʊ���Һ�����У��ձ��е�NH4Fe(SO4)2��Һ��������������TiO2���������ⶨ�����Ӱ��_________________________�����ƫ�ߡ�����ƫ�͡�����Ӱ�족��
��1�������� ��TiCl4+(2+x) H2O= TiO2��xH2O+4HCl
�ۼ�������Ƿ�ϴ���ķ����ǣ�ȡ����ϴ��Һ������ϴ��Һ���ܽ�������Ƿ���Cl����
��2���ٺ˴Ź��������ڲ��л����к��ж�������ԭ�ӣ�����������л��ﺬ�к��ֻ�ѧ�������ţ����������ڲ��л�����Է���������������������Թ۲쵽���Ĵ�С�� d ��a c
����Һ��ɺ�ɫ��30���ڲ���ɫ
�ܸ��ݵ�ʧ�����غ㣬�У�1Ti3+��1Fe3+,��n(Fe3+)= n(Ti3+)= n(TiO2)=cV��10-3mol������������Ϊ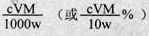 ��
��
��NH4Fe(SO4)2��Һ�����������ʵ���Ũ�ȼ�С�����ĵ�������ٷֺ���ƫ��
����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| cVM |
| 1000w |
| cVM |
| 10w |
| cVM |
| 1000w |
| cVM |
| 10w |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| cVM |
| 1000W |
| cVM |
| 10W |
| cVM |
| 1000W |
| cVM |
| 10W |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 TiO2?xH2O��+4HCl
TiO2?xH2O��+4HCl TiO2?xH2O��+4HCl
TiO2?xH2O��+4HCl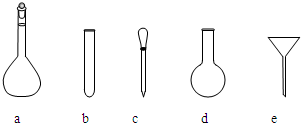
| cVM |
| 1000w |
| cVM |
| 10w |
| cVM |
| 1000w |
| cVM |
| 10w |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com