
| A��0.01mol��L��1��CH3COOH��pH=12��NaOH��Һ�������� |
| B��0.2 mol��L��1��CH3COOH��Һ��0.1 mol��L��1��NaOH��Һ�������� |
| C��CH3COOH��Һ��NaOH��Һ��Ϻ�������Һ��pH=7 |
| D��0.1 mol��L��1��CH3COOH��Һ������ʵ���Ũ�ȡ��������NaOH��Һ��� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ӧ��HA��Һ������ʣ�� |
| B��������NaA��ˮ��Һ��pH����С��7 |
| C��HA��Һ��NaOH��Һ��������ܲ���� |
| D��HA��Һ��c(H+)��NaOH��Һ��c(OH-)���ܲ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��c��NH4+��>c��Cl-��, c��OH-��> c��H+�� |
| B��c��NH4+��=c��Cl-��, c��OH-��= c��H+�� |
| C��c��Cl-�� > c��NH4+��, c��H+��> c��OH-�� |
| D��c��Cl-�� > c��NH4+��, c��OH-��> c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ������е�Ϊ77�棬��ʢ��10mL����ƿ��С�ĵμ�8��10��SOCl2���ɹ۲쵽����ˮ�ⷴӦ��Һ���а����ʹ��д̼�����ζ�������ݳ����������ʹ����Ʒ����Һ����ֽ��ɫ����������ƿ���Ȱ�����
������е�Ϊ77�棬��ʢ��10mL����ƿ��С�ĵμ�8��10��SOCl2���ɹ۲쵽����ˮ�ⷴӦ��Һ���а����ʹ��д̼�����ζ�������ݳ����������ʹ����Ʒ����Һ����ֽ��ɫ����������ƿ���Ȱ����� ʧ������Һ�еμ�AgNO3��Һ���в�����HNO3�İ�ɫ����״����������
ʧ������Һ�еμ�AgNO3��Һ���в�����HNO3�İ�ɫ����״���������� __��
__�� �յò�����ˮAlCl3������SOCl2��AlCl3��H2O ��Ϲ��ȣ��ɵõ���ˮAlCl3����ԭ���� __________________________ __________________��
�յò�����ˮAlCl3������SOCl2��AlCl3��H2O ��Ϲ��ȣ��ɵõ���ˮAlCl3����ԭ���� __________________________ __________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��c(K+)+c(H+)=c(S2��)+c(HS��)+c(OH��) |
| B��c(K+)+ c(S2��) = 0.3mol/L |
| C��c(K+)=c(S2��)+c(HS��)+c(H2S) |
| D��c(OH-)=c(H+)+c(HS��)+2c(H2S) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
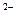 )< c(H2A)
)< c(H2A) c(OH�C) + c(A2�C)
c(OH�C) + c(A2�C)�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��FeCl 2��FeCl 3 | B��NaHCO3��Na2CO3 |
| C��NaAlO2��AlCl3 | D��Mg(HCO3)2��MgCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ʵ���� | HA���ʵ���Ũ�� ��mol��L-1�� | NaOH���ʵ���Ũ�� ��mol��L-1�� | �����Һ��pH |
| �� | 0.2 | 0.2 | pH=a |
| �� | C1 | 0.2 | pH=7 |
| �� | 0.1 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=10 |
 c(Na+)����Ũ�ȵĴ�С��ϵ�� ������ţ���A.ǰ�ߴ� B.���ߴ� C.һ���� D.���ж�
c(Na+)����Ũ�ȵĴ�С��ϵ�� ������ţ���A.ǰ�ߴ� B.���ߴ� C.һ���� D.���ж��鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com