
 BaCO3��+NH3��+2H2O ��2�֣�
BaCO3��+NH3��+2H2O ��2�֣�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H+��Al3+��K+��NO3һ | B��H+��Fe2+��OHһ��NO3һ |
| C��H+��Na+��Clһ��HCO3һ | D��H+��Na+��SO32һ��S2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ˮ�����c��OH����=10��14mol��L��1����Һ��CH3COO����SO42����Na����K�� |
| B���������ۺ����������������Һ��NH4����Na����NO3����Cl����SO42�� |
| C����ʹ pH ��ֽ������ɫ����Һ�У� S2һ��SO32����CO32����Na + |
| D����ɫ��Һ�У�K+��Na+��MnO4����SO42�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na+��NH4+��Al3+��H+ | B��Fe2+��Al3+��Na+��H+ |
| C��Na+��Fe3+��Al3+��H+ | D��Ag+��Fe3+��Na+��H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | NaOH�� �������� | �������� ���ʵ�����/g | ���������� �ʵ�����/g |
| A | 0.062(6.2%) | 19 | 152 |
| B | 0.062(6.2%) | 152 | 19 |
| C | 0.042(4.2%) | 1.2 | 9.4 |
| D | 0.042(4.2%) | 9.4 | 1.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ʹpH��ֽ�ʺ�ɫ����Һ��Fe2+��NO3����SO42����Na+ |
| B��pHֵΪ1����Һ��Cu2+��Na+��Mg2+��NO3- |
| C��ˮ���������c(H+)=10��13mol/L����Һ��K+��HCO3-��Br-��Ba2+ |
| D���ڼ������۷ų���������Һ��Na+��Cl����NH4+��NO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
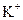 ��
��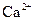 ��
��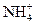 ��
��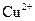 ��
��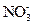 ��
��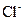 ��
�� ��
��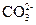 ���������е������֡�Ϊȷ������Һ����ɣ�����ֳ����ȷݣ���������ʵ�飺
���������е������֡�Ϊȷ������Һ����ɣ�����ֳ����ȷݣ���������ʵ�飺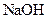 Ũ��Һ�����ȣ������������ڱ�״�������Ϊ4.48L��
Ũ��Һ�����ȣ������������ڱ�״�������Ϊ4.48L��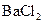 ��Һ500mL��ǡ�ÿ�������Һ�е�������ȫ��Ӧ�����˵�66.3g��������Һ��
��Һ500mL��ǡ�ÿ�������Һ�е�������ȫ��Ӧ�����˵�66.3g��������Һ��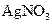 ��Һ650mL��ǡ�ÿ���ȫ��Ӧ���ݴˣ���ش��������⣨����Ӧ�����ӷ��ű�ʾ����
��Һ650mL��ǡ�ÿ���ȫ��Ӧ���ݴˣ���ش��������⣨����Ӧ�����ӷ��ű�ʾ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��OH-��Na+��K+��MnO4- | B��H+��Cl-��Ba2+��NO3- |
| C��K+��Cl-��Na+��SO42- | D��NH4+��Mg2+��Cl-��HCO3- |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com