
��һƿ�������Һ�����п��ܺ�NH4+��K+��Na+��Mg2+��Ba2+��Al3+��Fe3+��Cl-��I-��NO3-��CO32-��SO42-��ȡ����Һ��������ʵ�飺
��1��ȡpH��ֽ���飬������Һ��ǿ���ԣ��ų�__________���Ӵ��ڡ�
��2��ȡ��������Һ����������CCl4������������ˮ������CCl4����Ϻ�ɫ���ų�____���ӵĴ��ڡ�
��3����ȡ������Һ����NaOH��Һ��ʹ��Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ���Һ����������������ų�__________���ӵĴ��ڡ�ȡ���ּ�����Һ����������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��4����ȡ��������������Һ��Na2CO3��Һ���а�ɫ�������ɣ�֤��__________���Ӵ��ڣ����ų�__________���Ӵ��ڡ�
��5����������ʵ����ʵȷ��������Һ�п϶����ڵ�������__________���϶������ڵ�������__________��������ȷ���Ƿ���ڵ�������________________��Ҫȷ�����е������ӿ����õ�ԭ���� ��
��1��CO32-����2��NO3-��Fe3+(��I-��H+����NO3-)����3��Mg2+��Fe3+��Al3+����4��Ba2+��SO42-��
��5��Ba2+��I-��NH4+��Mg2+��Al3+��Fe3+��NO3-��CO32-��SO42-��K+��Na+��Cl-����ɫ��Ӧ
��������
�����������1��ȡpH��ֽ���飬������Һ��ǿ���ԣ�����CO32-�����������»ᷢ����Ӧ����CO2���壬�����ܴ������ڣ������ų�CO32-����2��ȡ��������Һ����������CCl4������������ˮ������CCl4����Ϻ�ɫ��֤������I-����I-���������������»���NO3-��Fe3+����������ԭ��Ӧ�����ܴ������棬��˿����ų�NO3-��Fe3+�Ĵ��ڣ���3����ȡ������Һ����NaOH��Һ��ʹ��Һ��������Ϊ���ԣ������ڼ��Ի�����Mg2+��Fe3+��Al3+�ᷢ����Ӧ�γɳ��������ڵμӹ����к͵μ���Ϻ���Һ����������������ų�Mg2+��Fe3+��Al3+�Ĵ��ڣ�ȡ���ּ�����Һ����������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������֤������Һ�к���NH4+����4����ȡ��������������Һ��Na2CO3��Һ���а�ɫ�������ɣ�֤��Ba2+���Ӵ��ڣ����ų���SO42-���Ӵ��ڣ���5����������ʵ����ʵȷ��������Һ�п϶����ڵ�������Ba2+��I-��NH4+���϶������ڵ�������Mg2+��Al3+��Fe3+��NO3-��CO32-��SO42-����ʵ����û���漰����������ȷ���Ƿ���ڵ�������K+��Na+��Cl-��Ҫȷ�����е������ӿ����õ�ԭ������ɫ��Ӧ������ʱ������ʻ�ɫ����֤������Na+��Ȼ������ɫ�ܲ����۲�������ɫ����֤������K+������Ͳ����ڸý��������ӡ�
���㣺�������ӵļ��鷽�������ӵĴ����빲���֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015�ӱ�����ij�ص���ѧͬ����ҵ������1�ս̰棩1.2.2�������ʵļ��� ���ͣ�ѡ����
�ܼ���������̼������ǡ�(����)
A��ʳ�� B������ˮ
C��ˮ D������ʯ��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ��ɽ�и߶���ѧ����ĩ���Ի�ѧ��B�����Ծ��������棩 ���ͣ�ѡ����
���и���������������ǿ����
A��H2SeO3 B��HMnO4 C�� H3BO3 D�� H3PO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ��ɽ�и߶���ѧ����ĩ���Ի�ѧ��A�����Ծ��������棩 ���ͣ�ѡ����
������ϵͳ�������������л���������ȷ����
A��2������4���һ����� B��3��4��4����������
C��1��2��4��������1������ D��2��3�����һ���1����ϩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ��ɽ�и߶���ѧ����ĩ���Ի�ѧ��A�����Ծ��������棩 ���ͣ�ѡ����
���Т�ʯ�͵ķ��� ��ú�ĸ��� ����֬��Ӳ�� �ܵ����ʵ����� �ݵ����ʵı��� �����ϻ����������������仯����
A���٢ڢ�B���٢�C���ۢܢ�D���٢ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ������ѧ���ڳ���⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
Ҫʹ����Ag+��Cu2+��Mg2+��Ba2+�����ӵ���Һ�е�������һ�γɳ���������������ѡ���Լ��������Լ���˳����ȷ����
A��H2SO4��HCl��K2S��NaOH��CO2 B��Na2SO4��NaCl��Na2S��NH3��H2O
C��NaCl��Na2SO4��H2S��NaOH D��Na2S��Na2SO4��NaCl��NaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ������ѧ���ڳ���⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
�����л����������ȷ����
A��2��2-����-3-��ϩ B��4��5-����-3-�һ�����
C��2��3��3-�������� D��2��3-����-4-�һ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ��˳����У������߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��CO2��H2��CO��ɵĻ�����壬��ͬ��ͬѹ����N2���ܶ���ͬ����û��������CO2��H2��CO�������Ϊ
A��29:8:13�� B��22:1:14 C��13:8:29 D��26:6:57
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�츣��ʡ���ݰ��и߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪0��1 mol/L��̼��������Һ��pHΪ8��4��������˵����ȷ���ǣ� ��
A����������NaOH���壬�����Ӻ�̼�������Ũ�Ⱦ�����
B��������Һ��ˮϡ�ͣ�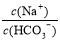 �ı�ֵ���ֲ���
�ı�ֵ���ֲ���
C��c(Na��)��c(H��)��c(HCO3�� )��c(CO32 ��)��c(OH��)
D��c(Na��)��c(HCO3�� )��2c(CO32 ��)��c(H2CO3)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com