
�Ȼ������ε�ⷨ�����Ȼ���Ϊԭ�ϣ��Լ��������������Ȼ��������MgCl2��KCl��CaCl2��Ϊ����ʽ��е����ȡ���ķ�����
��1���Ȼ������ε�ⷨ��Ҫ���ƴ�������������������Ҫ�ɷ�ΪAl2O3����������Fe2O3��SiO2�����ʣ�Ϊԭ��ͨ������;���ᴿ��������
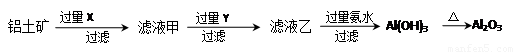
������д��X��Y�ijɷ� �� ��
����ͼ��ʾ��ʵ�����н��й��ˣ������е���������ֱ��� �� ��
��2���Ʊ���ˮ�Ȼ����ķ�ӦΪ��2Al2O3+6Cl2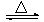 4AlCl3+3O2
4AlCl3+3O2
��Ϊ�ٽ��÷�Ӧ�Ľ��У�ʵ������������뽹̿����ԭ���� ��
�ܼ��뽹̿��Ļ�ѧ��Ӧ�ɱ�ʾΪAl2O3+C+Cl2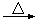 AlCl3+X����Ϊȷ������X�Ƿ��ǻ�����壬ijͬѧ��X����ͨ�����ȵ�����ͭ�ͳ����ʯ��ˮ���ٸ��������жϡ��ò����Ƿ���ȷ��������ȷ������ȷ�����жϣ� ����˵������
��
AlCl3+X����Ϊȷ������X�Ƿ��ǻ�����壬ijͬѧ��X����ͨ�����ȵ�����ͭ�ͳ����ʯ��ˮ���ٸ��������жϡ��ò����Ƿ���ȷ��������ȷ������ȷ�����жϣ� ����˵������
��
��3�����ڹ�ҵ��ͨ���õ��������������ʽ��ȡ��������������1�������ĵ�������������_________
������ڡ�����С�ڡ��������ڡ����Ȼ���������
��10�֣���1����NaOH��Һ��1�֣��� ϡ���ᣨ1�֣�
��û��ʹ�ò�������������ֽ��Ե����©����Ե��2�֣�
��2����̼��O2��Ӧ�������ڷ�Ӧ������У�2�֣�
�ܲ���ȷ��������������ͨ�����ȵ�CuO��ĩ������CO�ͻ�����CO2����������ȷ��ԭ���Ƿ���CO2����2�֣� ��3��С�ڣ�2�֣�
��������
�����������1������Һ���ܺͰ�ˮ��Ӧ��������������������˵����Һ���к��������ӣ�����YӦ�������ᡣ����Һ���к���AlO2����SiO32�������X��NaOH�����������������Ʋ���Ӧ�������õ��������������ƺ����ᷴӦ���ɹ�������������õ��Ȼ���������Ļ��Һ�����백����������������������
�ڸ���װ��ͼ��֪����װ���ǹ���װ�ã������Ҫ�Ĵ�������û��ʹ�ò����������������ֽ��Ե����©����Ե��
��2������Ϊ�ڼ��ȵ������£�̼��O2��Ӧ������CO��CO2������������Ũ�ȣ������ڷ�Ӧ������С�
�������ڼ��ȵ������£�CO�ܺ�����ͭ��Ӧ����CO2�����Խ�����������ͨ�����ȵ�CuO��ĩ������CO�ͻ�����CO2����������ȷ��ԭ���Ƿ���CO2�������Dz���ȷ�ġ�
��3������ԭ���غ��֪��1mol��������������2mol�Ȼ�����1mol��������������102g��2mol�Ȼ�����������2mol��133.5g/mol��267g������������������С���Ȼ�����������
���㣺������������ᴿ��ұ���������������Ʊ������˲��������������ƽ��״̬��Ӱ���Լ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ȼ������ε�ⷨ�����Ȼ���Ϊԭ�ϣ��Լ��������������Ȼ��������MgCl2��KCl��CaCl2��Ϊ����ʽ��е����ȡ���ķ�����
��1���Ȼ������ε�ⷨ��Ҫ���ƴ�������������������Ҫ�ɷ�ΪAl2O3����������Fe2O3��SiO2�����ʣ�Ϊԭ��ͨ������;���ᴿ��������

 |
������д��X��Y�ijɷ� �� ��
������ͼ��ʾ��ʵ�����н��й��ˣ�
�����е���������ֱ��� ��
��
��2���Ʊ���ˮ�Ȼ����ķ�ӦΪ��2Al2O3+6Cl2![]() 4AlCl3+3O2
4AlCl3+3O2
��Ϊ�ٽ��÷�Ӧ�Ľ��У�ʵ������������뽹̿����ԭ���� ��
�ܼ��뽹̿��Ļ�ѧ��Ӧ�ɱ�ʾΪAl2O3+C+Cl2![]() AlCl3+X����Ϊȷ������X�Ƿ��ǻ�����壬ijͬѧ��X����ͨ�����ȵ�����ͭ�ͳ����ʯ��ˮ���ٸ��������жϡ��ò����Ƿ���ȷ��������ȷ������ȷ�����жϣ� ����˵������ ��
AlCl3+X����Ϊȷ������X�Ƿ��ǻ�����壬ijͬѧ��X����ͨ�����ȵ�����ͭ�ͳ����ʯ��ˮ���ٸ��������жϡ��ò����Ƿ���ȷ��������ȷ������ȷ�����жϣ� ����˵������ ��
��3�����ڹ�ҵ��ͨ���õ��������������ʽ��ȡ��������������1�������ĵ������������� ������ڡ�����С�ڡ��������ڡ����Ȼ���������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com