
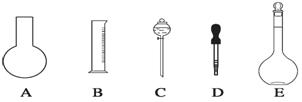
ʵ������Ҫ0.80 mol��L��1 NaOH��Һ475 mL ��0. 40 mol��L��1����500 mL��������������Һ����������ش��������⣺
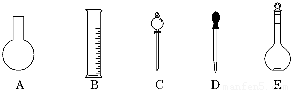
(1)��ͼ��ʾ��������������Һ�϶�����Ҫ����__________(����)������������Һ�����õ��IJ���������__________(����������)��
(2)����ƿ�����߱��Ĺ�����__________(����)��
A������һ�����ȷŨ�ȵı���Һ
B����ȡһ�������Һ��
C����������ƿ������µ����������Һ��
D��������Һ
E�����������ܽ��������
(3)���ݼ�����������ƽ��ȡNaOH������Ϊ________g����ʵ����������������ȷ��������ƿ������ˮϴ�Ӻ�δ�����������ҺŨ��__________0.80 mol��L��1(��������������С������������������ͬ)������δ����Һ��ȴ�Ͷ����ˣ���������ҺŨ��____________0.80 mol��L��1��
(4)���ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84 g��cm��3��Ũ��������Ϊ________mL(����������һλС��)�����ʵ������10 mL��15 mL��20 mL��50 mL����Ͳ�����ѡ��________mL����Ͳ��
����������(1)������Һ�ò���Բ����ƿ�ͷ�Һ©����
(2)����ƿֻ����������һ�����ȷŨ�ȵ���Һ��Ҳ������ȡ������ƿ�Ĺ����һ�µ�һ�������Һ�壬�����ܲ�������ƿ������µ����������Һ�壬���������ܽ����ʻ�������Һ��
(3)������500 mL������ƿ������0.80 mol��L��1 NaOH��Һ������Ҫ��ȡNaOH������Ϊ0.80 mol��L��1��0.5 L��40 g��mol��1��16.0 g������ƿδ���ﲻӰ����������Һ��Ũ�ȣ�δ����Һ��ȴ�Ͷ��ݻᵼ����ȴ����Һ�������С��Ũ�ȱ��
(4)����ҪŨ��������ΪV,1.84 g��cm��3��V��98%��0.40 mol��L��1��0.5 L��98 g��mol��1��V��10.9 mL��Ӧѡ��15 mL����Ͳ��
�𰸣�(1)A��C���ձ�����������(2)C��D��E��(3)16.0�����ڡ����ڡ�(4)10.9��15


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������Ҫ0.80 mol/L NaOH��Һ475 mL��0.40 mol/L������Һ500 mL��������������Һ����������ش��������⣺
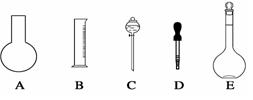
��1����ͼ��ʾ��������������Һ�϶�����Ҫ���� ������ţ�������������Һ�����õ��IJ��������� �����������ƣ���
��2�����ݼ�����������ƽ��ȡNaOH������Ϊ g����ʵ����������������ȷ��������ʱ�����ӿ̶��ߣ���������ҺŨ�� 0.8 mol/L������ڡ��������ڡ���С�ڡ�,��ͬ����������ʱ������������ˮ����������ƿ�⣬��������ҺŨ�� 0.8 mol/L��
��3�����ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84 g/cm3��Ũ��������Ϊ mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ����˫ʮ��ѧ2011�������һ���¿���ѧ���� ���ͣ������
��14�֣�ʵ������Ҫ0.80 mol/L NaOH��Һ475 mL��0.40 mol/L������Һ500 mL��������������Һ����������ش��������⣺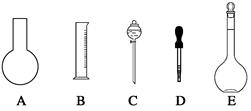
��1����ͼ��ʾ��������������Һ�϶�����Ҫ���� ������ţ�������������Һ�����õ��IJ��������� �����������ƣ���
��2�����в����У�����ƿ�����߱��Ĺ����� ������ţ���
| A������һ�����ȷŨ�ȵı���Һ |
| B����ȡһ�������Һ�� |
| C����������ƿ������µ����������Һ�� |
| D��ȷϡ��ijһŨ�ȵ���Һ |
 ���ߣ���������ҺŨ�� 0.8 mol/L������ڡ��������ڡ���С�ڡ�,��ͬ����������ʱ������������ˮ����������ƿ�⣬��������ҺŨ�� 0.8 mol/L��
���ߣ���������ҺŨ�� 0.8 mol/L������ڡ��������ڡ���С�ڡ�,��ͬ����������ʱ������������ˮ����������ƿ�⣬��������ҺŨ�� 0.8 mol/L���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ2010-2011ѧ�������һ���¿���ѧ���� ���ͣ������
��14�֣�ʵ������Ҫ0.80 mol/L NaOH��Һ475 mL��0.40 mol/L������Һ500 mL��������������Һ����������ش��������⣺
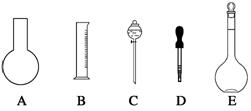
��1����ͼ��ʾ��������������Һ�϶�����Ҫ���� ������ţ�������������Һ�����õ��IJ��������� �����������ƣ���
��2�����в����У�����ƿ�����߱��Ĺ����� ������ţ���
A������һ�����ȷŨ�ȵı���Һ
B����ȡһ�������Һ��
C����������ƿ������µ����������Һ��
D��ȷϡ��ijһŨ�ȵ���Һ
E��������Һ
F�����������ܽ��������
��3�����ݼ�����������ƽ��ȡNaOH������Ϊ g����ʵ����������������ȷ��������ʱ�����ӿ̶��ߣ���������ҺŨ�� 0.8 mol/L������ڡ��������ڡ���С�ڡ�,��ͬ����������ʱ������������ˮ����������ƿ�⣬��������ҺŨ�� 0.8 mol/L��
��4�����ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84 g/cm3��Ũ��������Ϊ mL������������һλС���������ʵ������10 mL��15 mL��20 mL��50 mL��Ͳ��Ӧѡ�� mL��Ͳ��á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ�ʡ������ѧ�ڵڶ����¿���ѧ�Ծ� ���ͣ�ʵ����
��14�֣�ʵ������Ҫ0.80 mol/L NaOH��Һ475 mL��0.40 mol/L������Һ500 mL��������������Һ����������ش��������⣺
��1����ͼ��ʾ��������������Һ�϶�����Ҫ����___________������ţ�������������Һ�����õ��IJ���������________________________�����������ƣ���
��2�����ݼ�����������ƽ��ȡNaOH������Ϊ_______g�����ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84 g/cm3��Ũ��������Ϊ______mL��
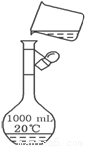
��3����ͼ��ijͬѧת����Һ��ʾ��ͼ��ͼ�еĴ��ڵĴ�����_______________________��
��4����ʵ����������������ȷ��������ʱ�����ӿ̶��ߣ���������ҺŨ��_____0.8 mol/L���������������ͬ����������ʱ������������ˮ����������ƿ�⣬��������ҺŨ��_____0.8 mol/L��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com