
 CH3CH2CN
CH3CH2CN CH3CH2COOH ������ӱ�ԭ��������Ӷ���һ��̼ԭ�ӣ�������̼������������¿�ͼ�ش�����
CH3CH2COOH ������ӱ�ԭ��������Ӷ���һ��̼ԭ�ӣ�������̼������������¿�ͼ�ش�����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
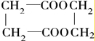
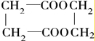
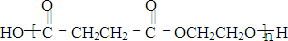
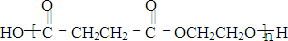
| Cu |
| �� |
| ���� |
| �� |
| Cu |
| �� |
| ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
CH3CH2Br![]() CH3CH2CN
CH3CH2CN![]() CH3CH2COOH
CH3CH2COOH
������ӱ�ԭ��������Ӷ���һ��̼ԭ�ӣ�������̼����������ұ߿�ͼ�ش����⣺
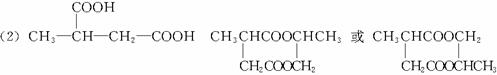
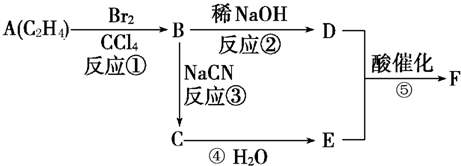
F�����к���8��ԭ����ɵĻ�״�ṹ��
(1)��Ӧ�٢ڢ�������ȡ����Ӧ����___________(�Ӧ����)��
(2)д���ṹ��ʽ��E___________��F___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
CH3CH2Br![]() CH3CH2CN
CH3CH2CN![]() CH3CH2COOH
CH3CH2COOH
������ӱ�ԭ��������Ӷ���һ��̼ԭ�ӣ�������̼������������¿�ͼ����������⣺
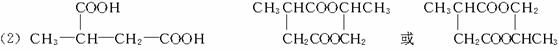
F�����к���8��ԭ����ɵĻ�״�ṹ��
��1����Ӧ�٢ڢ�������ȡ����Ӧ����____________(�Ӧ����)��
��2��д���ṹ��ʽ��E____________��F____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��������軯�Ʒ�Ӧ����ˮ����Եõ����
CH3CH2Br![]() CH3CH2CN
CH3CH2CN![]() CH3CH2COOH
CH3CH2COOH
������ӱ�ԭ��������Ӷ���һ��̼ԭ�ӣ�������̼����������ұ߿�ͼ�ش����⣺
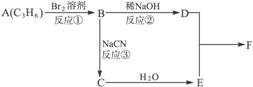
F�����к���8��ԭ����ɵĻ�״�ṹ��
(1)��Ӧ�٢ڢ�������ȡ����Ӧ����___________(�Ӧ����)��
(2)д���ṹ��ʽ����___________����___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��������軯�Ʒ�Ӧ����ˮ����Եõ�����
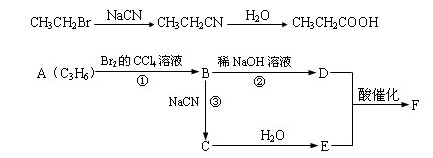
������ӱ�ԭ��������Ӷ���һ��̼ԭ�ӣ�������̼����
��������¿�ͼ�ش����⡣ͼ��F�����к���8��ԭ����ɵĻ�״�ṹ��
��1����Ӧ�٢ڢ�������ȡ����Ӧ����______________���Ӧ���ţ���
��2��д���ṹ��ʽ��E___________��F______________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com