
(1)��Ӧ�м�����Ҵ��ǹ����ģ���Ŀ����_____________��
(2)�ߵμӴ��ᣬ���������Ŀ����_____________��
���ֲ�Ʒ�پ����в��辫�ƣ�
(3)Ϊ��ȥ���еĴ��ᣬ�����Ʒ�м���___________(����ĸ)��
A.��ˮ�Ҵ� B.̼���Ʒ�ĩ C.��ˮ������
(4)�������м��뱥���Ȼ�����Һ�������룬��Ŀ����_____________��
(5)Ȼ���������м�����ˮ�����ƣ�����Ŀ����_____________��
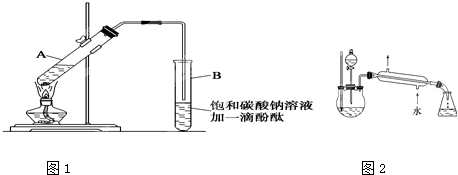
������������������Һ�������һ�����������ƿ�ڣ���������ȥ�ͷе���֣��ռ��е���76�桪
�����������ᷢ��������Ӧ�ǿ���ģ����ɵ�������ˮ�����ɴ����ᡣ
CH3COOH+CH3CH2OH![]() C2H5OOCCH3+H2O
C2H5OOCCH3+H2O
(����) (���)
Ϊ��ʹ��Ӧ���ϵ����������������ķ�����У���ȡ��ʹ�ù����Ҵ��Ͳ����������������Ĵ�ʩ�����ڷ�Ӧ��������ķе�ȽϽӽ���������ʱ�ɴ��������Ļ�����õ��������������и����������ʣ������Ҫ���ơ����ƹ�����ѡ�õĸ������ʼ�����Ӧ����Ҫ��ȥCH3COOH��CH3CH2OH��H2O��
�𰸣�(1)����Ӧ���Ҵ���Ũ�ȣ������ڷ�Ӧ���������������ķ������
(2)���Ӵ���Ũ�ȣ���С����������������Ũ�ȣ�������������Ӧ���������������ķ������
(3)B (4)��ȥ�ֲ�Ʒ�е��Ҵ� (5)��ȥ�ֲ�Ʒ�е�ˮ


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��� | �۵㣨�棩 | �е㣨�棩 | �ܶȣ�g/cm3�� |
| �ҡ��� | -117.0 | 78.0 | 0.79 |
| �ҡ��� | 16.6 | 117.9 | 1.05 |
| �������� | -83.6 | 77.5 | 0.90 |
| Ũ���ᣨ98%�� | - | 338.0 | 1.84 |
 CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
CH3COOC2H5Ϊ
�Խ���������⣺
(1)��Ӧ�м�����Ҵ��ǹ����ģ���Ŀ����___________________________________________��
(2)�ߵμӴ��ᣬ���������Ŀ����_________�����ֲ�Ʒ�پ����в��辫�ơ�
(3)Ϊ��ȥ���еĴ��ᣬ�����Ʒ�м���__________(������ѡ������)��
A.��ˮ�Ҵ� B.̼���Ʒ�ĩ C.��ˮ������
(4)�������м��뱥���Ȼ�����Һ�������룬��Ŀ����____________________��
(5)Ȼ���������м�����ˮ�����ƣ�����Ŀ����__________________________��
������������������Һ�������һ���������ƿ�ڣ���������ȥ�ͷе���֣��ռ��е���76��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.ˮϴ B.10%��NaOH��Һϴ
C.ˮϴ D.�ø�������� E.����
����������⣺
��д����ȡ�屽�Ļ�ѧ����ʽ��__________________________________________��
�ڲ���A����ҪĿ���ǣ�_______________________________________________��
�۲���B������Ӧ�����ӷ���ʽΪ��_____________________________________��
�ܲ���A��B��C��Ӧ��___________�������������н��У�������ʵ�������Ҫ������___________��___________�ֲ㡢___________��
�ݲ���E��Ŀ���ǣ�_______________________________________________________��
��2����֪�Ҵ����Ժ��Ȼ��Ʒ�Ӧ��������ˮ��CaCl2��6C2H5OH���йص��л��Լ��ķе����£�
CH3COOC2H5��77.1 �棻C2H5OH��78.3 ��
C2H5OC2H5�����ѣ���34.5 �棻CH3COOH��118 ��
ʵ���Һϳ����������ֲ�Ʒ�IJ������£�
������A�ڽ��������Ҵ�������Ũ�����ϣ�Ȼ��Һ©���ߵμӴ��ᡢ�������õ������Ҵ������ѡ������ˮ�����������ֲ�Ʒ��
������A��������____________________��
�ڷ�Ӧ�м�����Ҵ��ǹ����ģ���Ŀ���ǣ�__________________________________��
�۱ߵμӴ��ᡢ���������Ŀ���ǣ�________________________________________��
���ֲ�Ʒ�پ����в��辫�ƣ�
��Ϊ��ȥ���еĴ��ᣬ�����Ʒ�м��루����ĸ��___________��
A.��ˮ�Ҵ� B.̼���Ʒ�ĩ C.��ˮ������
���������м��뱥���Ȼ�����Һ�������롣��Ŀ���ǣ�__________________________��
��Ȼ���������м�����ˮ�����ƣ�����Ŀ���ǣ�_________________________________��
������������������Һ�������һ���������A�ڣ���������ȥ�ͷе���֣��ռ��г�76 �桪78 ��֮�����ּ��á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ����һ�и߶�10���¿���ѧ�Ծ����������� ���ͣ�ʵ����
��֪�Ҵ����Ժ��Ȼ��Ʒ�Ӧ��������ˮ��CaCl2��6C2H5OH���йص��л��Լ��ķе����£�CH3COOC2H5 77.1�� C2H5OC2H5(����) 34.5�� C2H5OH 78.3�� CH3COOH 118�� ʵ���Һϳ����������ֲ�Ʒ�IJ������£���������ƿ�ڽ��������Ҵ�������Ũ�����ϣ�Ȼ��Һ©���ߵμӴ��ᡢ�������õ������Ҵ������ѡ������ˮ�����������ֲ�Ʒ��
(1)��Ӧ�м����Ҵ��ǹ����ģ���Ŀ���ǣ� ��
���ֲ�Ʒ�پ����в��辫�ƣ�
(2)Ϊ��ȥ���еĴ��ᣬ�����Ʒ�м���__________������ĸ����
A ��ˮ�Ҵ� B ̼���Ʒ�ĩ C ��ˮ������
(3)�������м��뱥���Ȼ�����Һ������Ŀ���ǣ� ��
(4)Ȼ���������м�����ˮ�����ƣ�����Ŀ���ǣ� ��
������������������Һ�������һ���������ƿ�ڣ���������ȥ�ͷе���֣��ռ�76~78��г�֮�����ּ��á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭ʡ�߶�10���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
��֪�Ҵ����Ժ��Ȼ��Ʒ�Ӧ��������ˮ��CaCl2��6C2H5OH���йص��л��Լ��ķе����£�CH3COOC2H5 77.1�� C2H5OC2H5(����) 34.5�� C2H5OH 78.3�� CH3COOH 118�� ʵ���Һϳ����������ֲ�Ʒ�IJ������£���������ƿ�ڽ��������Ҵ�������Ũ�����ϣ�Ȼ��Һ©���ߵμӴ��ᡢ�������õ������Ҵ������ѡ������ˮ�����������ֲ�Ʒ��
(1)��Ӧ�м����Ҵ��ǹ����ģ���Ŀ���ǣ� ��
���ֲ�Ʒ�پ����в��辫�ƣ�
(2)Ϊ��ȥ���еĴ��ᣬ�����Ʒ�м���__________������ĸ����
A ��ˮ�Ҵ� B ̼���Ʒ�ĩ C ��ˮ������
(3)�������м��뱥���Ȼ�����Һ������Ŀ���ǣ� ��
(4)Ȼ���������м�����ˮ�����ƣ�����Ŀ���ǣ� ��
������������������Һ�������һ���������ƿ�ڣ���������ȥ�ͷе���֣��ռ�76~78��г�֮�����ּ��á�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com