
(1)
(2)�����ϱ�ֵ�ܷ�ȷ���ô��ķ���ʽ��___________,��ԭ����______________________��
(3)������ö�Ԫ��������һ���ǻ�����һ��±ԭ�ӣ����õ���±���ﶼֻ��һ�֣���д���ñ��Ͷ�Ԫ���Ľṹ��ʽ____________________________________________��
(1)0.125 0.30 0.1 5��12��4
(2)�� ��Ϊ��ʵ��ʽ��Hԭ�Ӹ����Ѵﱥ��
(3)C(CH2OH)4
������(1)CO2����ʯ�����գ��������![]() =0.3 mol,�л�����������ȥC��H����֮�ͼ�ΪO��������(2)ʵ��ʽΪC5H12O4��Hԭ�����Ѵﱥ�ͣ�����2n+2����ʵ��ʽ��Ϊ����ʽ��(3)±����ֻ��һ�֣���һ�ֶԳƽṹ���ô�Ϊ
=0.3 mol,�л�����������ȥC��H����֮�ͼ�ΪO��������(2)ʵ��ʽΪC5H12O4��Hԭ�����Ѵﱥ�ͣ�����2n+2����ʵ��ʽ��Ϊ����ʽ��(3)±����ֻ��һ�֣���һ�ֶԳƽṹ���ô�Ϊ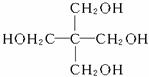 ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��
��2�������ϱ�ֵ�ܷ�ȷ���ô��ķ���ʽ��_________________����ԭ����_________________��
��3������ö�Ԫ��������һ���ǻ�����һ��±ԭ�ӣ����õ���±���ﶼֻ��һ�֣���д���ñ��Ͷ�Ԫ���Ľṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)
(2)�����ϱ�ֵ_____________(��ܡ����ܡ�)ȷ���ô��ķ���ʽ����ԭ����_________________________________________________________________��
(3)������ö�Ԫ��������һ���ǻ�����һ��±ԭ�ӣ����õ���±���ﶼֻ��һ�֣���д���ñ��Ͷ�Ԫ���Ľṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)3.40 g����C��H��O���ʵ����ֱ�Ϊ��C__________mol��O__________mol���ô���C��H��O��ԭ����֮��Ϊ__________��
(2)�����ϱ�ֵ�ܷ�ȷ���ô��ķ���ʽ��__________����ԭ����____________________��
(3)����ö�Ԫ��������һ���ǻ�����һ��±ԭ�ӣ����õ���±���ﶼֻ��һ�֣���д���ñ��Ͷ�Ԫ���Ľṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��3.40 g����C��H��O���ʵ����ֱ�Ϊ��C_______mol��H_______mol��O_______mol���ô���C��H��Oԭ�Ӹ���֮��Ϊ______________��
��2�������ϱ�ֵ�ܷ�ȷ���ô��ķ���ʽ��_______��ԭ����___________________________��
��3��������ö�Ԫ��������һ���ǻ�����һ��±ԭ�ӣ����õ���±����ֻ��һ�֣���д���ñ��Ͷ�Ԫ���Ľṹ��ʽ��___________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com