
ҽ���Ȼ��ƿ����ڲ��ƿ������ȣ��Թ�ҵ̼���(������Na+��Al3+��Fe3+������)����ҽ�ö�ˮ���Ȼ���(CaCl2��2H2O����������Ϊ97.3��103.0��)����������Ϊ��
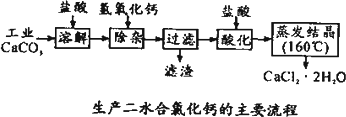
(1)CaCO3��
���ᷴӦ�����ӷ���ʽ________��(2)����ʱ���õIJ����������ձ������������________��������Ҫ�ɷֵĻ�ѧʽ________��
(3)�ữʱ�������Ŀ��Ϊ����________���ڷ�ֹCa2+������ʱ����ˮ�⣮
(4)Ϊʲô�����ᾧҪ������160�棺________��
(5)Ϊ�ⶨ��Ʒ��CaCl2��2H2O�ĺ�������ȡ0.7522 g��Ʒ�����250 mL��Һ���ֱ�ȡ����Һ25.00 mL��������ƿ�У���0.04 mol/LAgNO3��Һ�������εζ�������AgNO3��Һ��ƽ�����Ϊ20.39 mL��
��ͨ�����㣬��Ʒ�к�CaCl2��2H2O����������________��
����������ƷCaCl2��2H2O����������ƫ��(��������ʵ�����)�����ܵ�ԭ��֮һΪ________��
 ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�ҽ���Ȼ��ƿ����ڲ��ƿ������ȣ��Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+�����ʣ�����ҽ�ö�ˮ���Ȼ��ƣ�CaCl2 ��2H2O����������Ϊ97.3��103.0%������������Ϊ��
��1��CaCO3�����ᷴӦ�����ӷ���ʽ ��
��2������ʱ���õIJ����������ձ������������ ��������Ҫ�ɷֵĻ�ѧʽ ��
��3���ữʱ�������Ŀ��Ϊ��
�� ��
�ڷ�ֹCa2+ ������ʱ����ˮ�⡣
��4��Ϊʲô�����ᾧҪ������160�棺 ��
��5��Ϊ�ⶨ��Ʒ��CaCl2 ��2H2O�ĺ�������ȡ0.7522g��Ʒ�����250mL��Һ���ֱ�ȡ����Һ25.00mL��������ƿ�У���0.04mol/LAgNO3��Һ�������εζ������� AgNO3��Һ��ƽ�����Ϊ20.39mL��
��ͨ�����㣬��Ʒ�к�CaCl2 ��2H2O���������� ��
����������Ʒ CaCl2 ��2H2O����������ƫ�ߣ���������ʵ���������ܵ�ԭ��֮һΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��2012ѧ��㶫ʡ����ѧ���������ܲ⻯ѧ�Ծ� ���ͣ�ʵ����
��16�֣�ҽ���Ȼ��ƿ����ڲ��ƿ������ȣ��Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+�����ʣ�����ҽ�ö�ˮ���Ȼ��ƣ�CaCl2 ��2H2O����������Ϊ97.3��103.0%������������Ϊ��
(�����õ���ԭ������Cl 35.5 Ca 40 O 16 )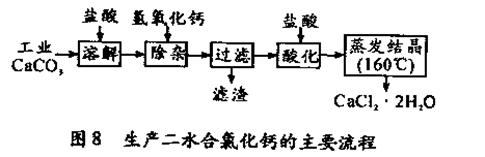
��1��CaCO3�����ᷴӦ�����ӷ���ʽ ��
��2������ʱ���õIJ����������ձ������������ ��������Ҫ�ɷֵĻ�ѧʽ ��
��3���ữʱ�������Ŀ��Ϊ��
�� ��
�ڷ�ֹCa2+ ������ʱ����ˮ�⡣
��4��Ϊʲô�����ᾧҪ������160�棺  ��
��
��5��Ϊ�ⶨ��Ʒ��CaCl2 ��2H2O�ĺ�������ȡ0.7522g��Ʒ�����250mL��Һ���ֱ�ȡ����Һ25.00mL��������ƿ�У���0.04mol/LAgNO3��Һ�������εζ������� AgNO3��Һ��ƽ�����Ϊ20.39mL��
��ͨ�����㣬��Ʒ�к�CaCl2 ��2H2O���������� ����ֻд����ʽ������������
����������Ʒ CaCl2 ��2H2O����������ƫ�ߣ���������ʵ���������ܵ�ԭ��֮һΪ  ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ�ٺ��а�����ѧ������ѧ�ڵ�һ���¿���ѧ����� ���ͣ�ʵ����
��14�֣�ҽ���Ȼ��� �����ڲ��ƿ������ȣ��Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+�����ʣ�����ҽ�ö�ˮ���Ȼ��ƣ�CaCl2 ��2H2O����������Ϊ97.3��103.0%������������Ϊ��
�����ڲ��ƿ������ȣ��Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+�����ʣ�����ҽ�ö�ˮ���Ȼ��ƣ�CaCl2 ��2H2O����������Ϊ97.3��103.0%������������Ϊ�� 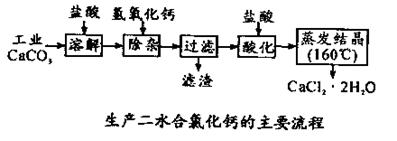
��1��CaCO3�����ᷴӦ�����ӷ���ʽ ��
��2������ʱ���õIJ����������ձ������������ ��������Ҫ�ɷֵĻ�ѧʽ ��
��3���ữʱ�������Ŀ��Ϊ��
�� ��
�ڷ�ֹCa2+ ������ʱ����ˮ�⡣
��4��Ϊʲô���� �ᾧҪ������160�棺 ��
�ᾧҪ������160�棺 ��
��5��Ϊ�ⶨ��Ʒ��CaCl2 ��2H2O�ĺ�������ȡ0.7522g��Ʒ�����250mL��Һ���ֱ�ȡ����Һ25.00mL��������ƿ�У���0.04mol/LAgNO3��Һ�������εζ������� AgNO3��Һ��ƽ�����Ϊ20.39mL��
��ͨ�����㣬��Ʒ�к�CaCl2 ��2H2O���������� ��
����������Ʒ CaCl2 ��2H2O����������ƫ�ߣ���������ʵ���������ܵ�ԭ��֮һΪ ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ�ٺ��и�����ѧ�ڵ�һ���¿���ѧ����� ���ͣ�ʵ����
��14����ҽ���Ȼ��ƿ����ڲ��ƿ������ȣ��Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+�����ʣ�����ҽ�ö�ˮ���Ȼ��ƣ�CaCl2 ��2H2O����������Ϊ97.3��103.0%������������Ϊ��
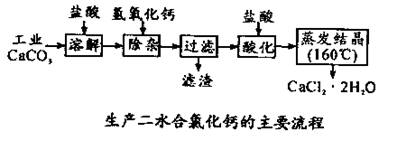
��1��CaCO3�����ᷴӦ�����ӷ���ʽ ��
��2������ʱ���õIJ����������ձ������������ ��������Ҫ�ɷֵĻ�ѧʽ ��
��3���ữʱ�������Ŀ��Ϊ��
�� ��
�ڷ�ֹCa2+ ������ʱ����ˮ�⡣
��4��Ϊʲô�����ᾧҪ������160�棺 ��
��5��Ϊ�ⶨ��Ʒ��CaCl2 ��2H2O�ĺ�������ȡ0.7522g��Ʒ�����250mL��Һ���ֱ�ȡ����Һ25.00mL��������ƿ�У���0.04mol/LAgNO3��Һ�������εζ������� AgNO3��Һ��ƽ�����Ϊ20.39mL��
��ͨ�����㣬��Ʒ�к�CaCl2 ��2H2O���������� ��
����������Ʒ CaCl2 ��2H2O����������ƫ�ߣ���������ʵ���������ܵ�ԭ��֮һΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��㶫ʡ���������ܲ⻯ѧ�Ծ� ���ͣ�ʵ����
��16�֣�ҽ���Ȼ��ƿ����ڲ��ƿ������ȣ��Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+�����ʣ�����ҽ�ö�ˮ���Ȼ��ƣ�CaCl2 ��2H2O����������Ϊ97.3��103.0%������������Ϊ��
(�����õ���ԭ������Cl 35.5 Ca 40 O 16 )
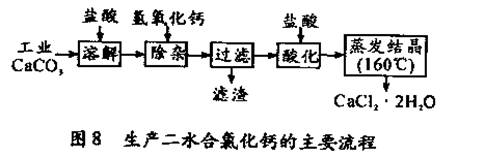
��1��CaCO3�����ᷴӦ�����ӷ���ʽ ��
��2������ʱ���õIJ����������ձ������������ ��������Ҫ�ɷֵĻ�ѧʽ ��
��3���ữʱ�������Ŀ��Ϊ��
�� ��
�ڷ�ֹCa2+ ������ʱ����ˮ�⡣
��4��Ϊʲô�����ᾧҪ������160�棺 ��
��5��Ϊ�ⶨ��Ʒ��CaCl2 ��2H2O�ĺ�������ȡ0.7522g��Ʒ�����250mL��Һ���ֱ�ȡ����Һ25.00mL��������ƿ�У���0.04mol/LAgNO3��Һ�������εζ������� AgNO3��Һ��ƽ�����Ϊ20.39mL��
��ͨ�����㣬��Ʒ�к�CaCl2 ��2H2O���������� ����ֻд����ʽ������������
����������Ʒ CaCl2 ��2H2O����������ƫ�ߣ���������ʵ���������ܵ�ԭ��֮һΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com