��2012?������ģ����ͼ�Ǻϳɰ������ð���ȡ���ᡢ����ļ�Ҫ����ʾ��ͼ��
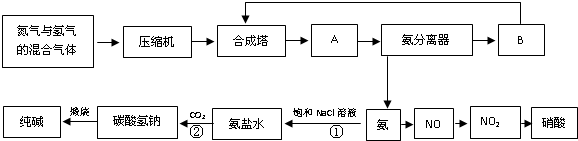
��1���豸A��������
������
������
���豸B��������
ѭ��ѹ����
ѭ��ѹ����
��
��2���йغϳɰ���ҵ��˵���У���ȷ����
B
B
��
A���Ӻϳ��������Ļ�����壬����NH
3ֻռ15%�������������Ĺ�����Ч�ʶ��ܵ�
B�����ڰ���Һ����N
2��H
2��ѭ��ʹ�ã�����������˵���IJ��ʺܸ�
C���ϳɰ���ҵ�ķ�Ӧ�¶ȿ�����500�����ң�Ŀ����ʹ��ѧƽ��������Ӧ�����ƶ�
D���ϳɰ������õ�ѹǿ��2��10
7��5��10
7Pa�����ڸ�ѹǿ������ý�Ļ������
��3���ڴ�����������У����̢�����̢ڵ�˳���ܷ�ߵ���
����
����
����������
��ΪCO2��ˮ���ܽ�Ƚ�С��˳��ߵ���ò����ϸ�Ũ�ȵ�HCO3-������û��NaHCO3����
��ΪCO2��ˮ���ܽ�Ƚ�С��˳��ߵ���ò����ϸ�Ũ�ȵ�HCO3-������û��NaHCO3����
������Ʒ�����к���̼�����ƣ��ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�����������������̼�����Ƶ����������ɱ�ʾΪ��
��ע����ı���ʽ�����õ��йط��ŵĺ��壩
��4�����᳧��β���к���NO��NO
2����Ⱦ������ø���ȼ�����еļ���Ƚ����������ﻹԭΪ������Ҫ�ɷֶ���ȥ��д��������NO
2��Ӧ�Ļ�ѧ��Ӧ����ʽ��
CH4+2NO2�TN2+CO2+2H2O
CH4+2NO2�TN2+CO2+2H2O
��
���������з�Ӧ�ķ���������2NO
2+2NaOH��NaNO
3+NaNO
2+H
2O��NO+NO
2+2NaOH��2NaNO
2+H
2O�����б�״����NO��NO
2�Ļ����ǡ����50mL 2.0mol?L
-1��NaOH��Һ��Ӧ��ȫ��������NaNO
2��NaNO
3�����ʵ����ı�ֵΪ4��1�����ڻ��������NO������������Ϊ
0.3
0.3
��



 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�

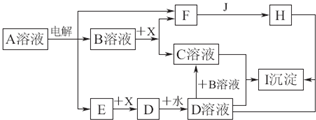
 A��B��D��E��GΪ������Ԫ�أ���ԭ��������������AԪ�������ڱ���ԭ�Ӱ뾶��С��BԪ�ص���������ϼۺ�������ϼ۵ľ���ֵ��ȣ�EԪ�ص�ԭ�������������Ǵ�����3����E��G���γ����ӻ�����GE��
A��B��D��E��GΪ������Ԫ�أ���ԭ��������������AԪ�������ڱ���ԭ�Ӱ뾶��С��BԪ�ص���������ϼۺ�������ϼ۵ľ���ֵ��ȣ�EԪ�ص�ԭ�������������Ǵ�����3����E��G���γ����ӻ�����GE��
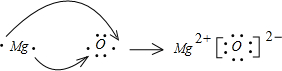
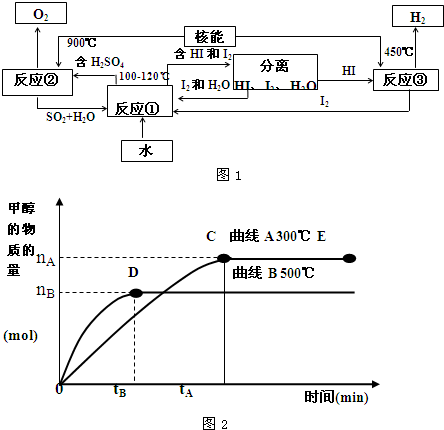
 ��һ���¶��£�������ijһ��Ӧ��M��N�����ʵ����淴Ӧʱ��仯��������ͼ������������ȷ���ǣ�������
��һ���¶��£�������ijһ��Ӧ��M��N�����ʵ����淴Ӧʱ��仯��������ͼ������������ȷ���ǣ�������