
(11��)ij����С�����������ʵ�鷽����֤Ag��ŨHNO3��Ӧ�Ĺ����п��ܲ���NO����ʵ������ͼ���£�
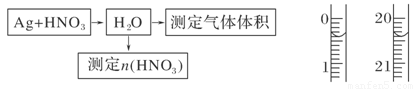
(1)�ⶨ��������ʵ���
��Ӧ��������ͼBװ��������100 mL��Һ��ȡ��25.00 mL��Һ����0.1 mol��L��1��NaOH��Һ�ζ����÷�̪��ָʾ�����ζ�ǰ��ĵζ�����Һ���λ������ͼ��ʾ����B������������������ʵ���Ϊ________mol����Ag��Ũ���ᷴӦ���������ɵ�NO2���Ϊ________mL��
(2)�ⶨNO�����
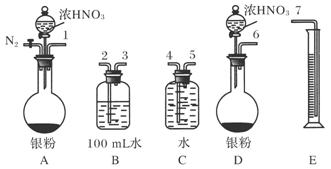
�ٴ���ͼ��ʾ��װ���У�����ΪӦѡ��________װ�ý���Ag��Ũ���ᷴӦʵ�飬ѡ�õ�������______________________________________________________________________
________________________________________________________________________��
��ѡ����ͼ��ʾ�������һ�������ⶨ����NO�����װ�ã������������˳����________(������ܿڱ��)�����ڲⶨNO�����ʱ������Ͳ��ˮ��Һ��ȼ���ƿ��Һ��Ҫ�ͣ���ʱӦ����Ͳ��λ��________(ѡ��½��������ߡ�)���Ա�֤��Ͳ�е�Һ���뼯��ƿ�е�Һ���ƽ��
(3)����ɷַ���
��ʵ����NO�����Ϊ112.0 mL(�����㵽��״��)����Ag��Ũ���ᷴӦ�Ĺ�����________(��С���û�С�)NO�����������жϵ�������
________________________________________________________________________
________________________________________________________________________��
(1)0.008 268.8
(2)��A ��ΪAװ�ÿ���ͨ��N2��װ���еĿ����ž�����ֹNO��������O2���� ��123547(1547����Ҳ��)��������
(3)�� ��ΪNO2��ˮ��Ӧ���ɵ�NO�����С���ռ�����NO�����(89.6<112.0)
��������(1)Bװ���з����ķ�Ӧ��3NO2��H2O===2HNO3��NO����NaOH��Һ�ζ�����HNO3����n(HNO3)��4n(NaOH)��4��0.1 mol��L��1��(20.40��0.40)��10��3 L��0.008 mol��������NO2�����Ϊ��0.008 mol����22.4 L/mol��103 mL/L��268.8 mL��(2)��ѡAװ�ã���ͨ��N2��װ���еĿ����ž�����Ӧ��֤��Ͳ�ڵ�Һ���뼯��ƿ�е�Һ����ƽ��ʹ��Ͳλ�����ߡ�(3)��(1)֪����n(NO)��n(HNO3)��0.004 mol����Ϊ89.6 mL�����ռ���NOΪ112.0 mL����Ag��ŨHNO3��Ӧ�����в�����NO��


 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(11��)ij����С�����������ʵ�鷽����֤Ag��ŨHNO3��Ӧ�Ĺ����п��ܲ���NO����ʵ������ͼ���£�
(1)�ⶨ��������ʵ���
��Ӧ��������ͼBװ��������100 mL��Һ��ȡ��25.00 mL��Һ����0.1 mol��L��1��NaOH��Һ�ζ����÷�̪��ָʾ�����ζ�ǰ��ĵζ�����Һ���λ������ͼ��ʾ����B������������������ʵ���Ϊ________mol����Ag��Ũ���ᷴӦ���������ɵ�NO2���Ϊ________mL��
(2)�ⶨNO�����
�ٴ���ͼ��ʾ��װ���У�����ΪӦѡ��________װ�ý���Ag��Ũ���ᷴӦʵ�飬ѡ�õ�������______________________________________________________________________
________________________________________________________________________��
��ѡ����ͼ��ʾ�������һ�������ⶨ����NO�����װ�ã������������˳����________(������ܿڱ��)�����ڲⶨNO�����ʱ������Ͳ��ˮ��Һ��ȼ���ƿ��Һ��Ҫ�ͣ���ʱӦ����Ͳ��λ��________(ѡ��½��������ߡ�)���Ա�֤��Ͳ�е�Һ���뼯��ƿ�е�Һ���ƽ��
(3)����ɷַ���
��ʵ����NO�����Ϊ112.0 mL(�����㵽��״��)����Ag��Ũ���ᷴӦ�Ĺ�����________(��С���û�С�)NO�����������жϵ�������
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(11��)ij����С������ȡ�������ƺ��Ȼ��ƵĻ����Һ��Ϊ��ߴ������ƺ�����������ͼ��ʾװ�á�ͼ��ƿ��ʢ����ʳ��ˮ��ƿ��ʢŨ���ᣬ��Һ©��A��ʢŨ���ᡣ(��������ʾ��Cl2��NaOH�ڲ�ͬ�¶��£����ﲻͬ���ڽϸ��¶���������NaClO3)
�Իش�
(1)��ƿB��ʢ________���Թ�C��ʢ________��
(2)��ͬѧ��Ϊ����ʡȥijЩװ�ã�����Ϊ������
���ܷ�ʡȥ��װ��________(��ܡ����ܡ�)��������
________________________________________________________________________��
���ܷ�ʡȥ��װ��________(��ܡ����ܡ�)��������
_____________________________________________________________________________________��
(3)��ͬѧ��Ϊ����������ijЩװ�ã�����Ϊ������________(���Ҫ������Ҫ��)���������Ϊ��Ҫ����ָ����װ�õ�������_______________________________________________��
(4)��װ���б�ˮ��������____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�������ѧ�ڻ�ѧһ�ָ�ϰ���Ӻ�ˮ�л�û�ѧ���ʡ�ר���ۺϲ��ԣ��ս̰棩 ���ͣ�ʵ����
(11��)ij����С������ȡ�������ƺ��Ȼ��ƵĻ����Һ��Ϊ��ߴ������ƺ�����������ͼ��ʾװ�á�ͼ��ƿ��ʢ����ʳ��ˮ��ƿ��ʢŨ���ᣬ��Һ©��A��ʢŨ���ᡣ(��������ʾ��Cl2��NaOH�ڲ�ͬ�¶��£����ﲻͬ���ڽϸ��¶���������NaClO3)
�Իش�
(1)��ƿB��ʢ________���Թ�C��ʢ________��
(2)��ͬѧ��Ϊ����ʡȥijЩװ�ã�����Ϊ������
���ܷ�ʡȥ��װ��________(��ܡ����ܡ�)��������
________________________________________________________________________��
���ܷ�ʡȥ��װ��________(��ܡ����ܡ�)��������
_____________________________________________________________________________________��
(3)��ͬѧ��Ϊ����������ijЩװ�ã�����Ϊ������________(���Ҫ������Ҫ��)���������Ϊ��Ҫ����ָ����װ�õ�������_______________________________________________��
(4)��װ���б�ˮ��������____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�������ѧ�ڻ�ѧһ�ָ�ϰ�����Ϳɳ�����չ��ר���ۺϲ��ԣ��ս̰棩 ���ͣ�ʵ����
(11��)ij����С�����������ʵ�鷽����֤Ag��ŨHNO3��Ӧ�Ĺ����п��ܲ���NO����ʵ������ͼ���£�
(1)�ⶨ��������ʵ���
��Ӧ��������ͼBװ��������100 mL��Һ��ȡ��25.00 mL��Һ����0.1 mol��L��1��NaOH��Һ�ζ����÷�̪��ָʾ�����ζ�ǰ��ĵζ�����Һ���λ������ͼ��ʾ����B������������������ʵ���Ϊ________mol����Ag��Ũ���ᷴӦ���������ɵ�NO2���Ϊ________mL��
(2)�ⶨNO�����
�ٴ���ͼ��ʾ��װ���У�����ΪӦѡ��________װ�ý���Ag��Ũ���ᷴӦʵ�飬ѡ�õ�������______________________________________________________________________
________________________________________________________________________��
��ѡ����ͼ��ʾ�������һ�������ⶨ����NO�����װ�ã������������˳����________(������ܿڱ��)�����ڲⶨNO�����ʱ������Ͳ��ˮ��Һ��ȼ���ƿ��Һ��Ҫ�ͣ���ʱӦ����Ͳ��λ��________(ѡ��½��������ߡ�)���Ա�֤��Ͳ�е�Һ���뼯��ƿ�е�Һ���ƽ��
(3)����ɷַ���
��ʵ����NO�����Ϊ112.0 mL(�����㵽��״��)����Ag��Ũ���ᷴӦ�Ĺ�����________(��С���û�С�)NO�����������жϵ�������
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�������ѧ�ڻ�ѧһ�ָ�ϰ���Ӻ�ˮ�л�û�ѧ���ʡ�ר���ۺϲ��ԣ��ս̰棩 ���ͣ�ʵ����
(11��)ij����С������ȡ�������ƺ��Ȼ��ƵĻ����Һ��Ϊ��ߴ������ƺ�����������ͼ��ʾװ�á�ͼ��ƿ��ʢ����ʳ��ˮ��ƿ��ʢŨ���ᣬ��Һ©��A��ʢŨ���ᡣ(��������ʾ��Cl2��NaOH�ڲ�ͬ�¶��£����ﲻͬ���ڽϸ��¶���������NaClO3)
�Իش�
(1)��ƿB��ʢ________���Թ�C��ʢ________��
(2)��ͬѧ��Ϊ����ʡȥijЩװ�ã�����Ϊ������
���ܷ�ʡȥ��װ��________(��ܡ����ܡ�)��������
________________________________________________________________________��

���ܷ�ʡȥ��װ��________(��ܡ����ܡ�)��������
_____________________________________________________________________________________��
(3)��ͬѧ��Ϊ����������ijЩװ�ã�����Ϊ������________(���Ҫ������Ҫ��)���������Ϊ��Ҫ����ָ����װ�õ�������_______________________________________________��
(4)��װ���б�ˮ��������____________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com