
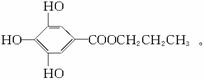
(1)��ûʳ��������īˮ��Ҫ������___________����������(�����)��
A.�� B.�� C.��֬ D.����
(2)ûʳ����������п��������ã���Ŀǰ�㷺Ӧ�õ�ʳƷ���Ӽ�����ṹ��ʽΪ______________________________________________________��
(3)�Ჴ�����Ƕ��ǻ��������봼�γɵ��������ǹ�������ʹ�õ�ʳƷ���������Ჴ�����ķ���ʽΪ_____________________________���䱽��ֻ�롪OH�͡�COOR����ȡ����ֱ��������ͬ���칹����_____________�֡�
(4)д���Ჴ������������������Һ���ȷ�Ӧ�Ļ�ѧ����ʽ��______________________��
�������������л���ûʳ���ᡢ�Ჴ����Ϊ�زģ������л���ṹʽ��ʶ�𡢷���ʽ����д��ͬ���칹���ȷ���������ŵ����ʼ���Ҫ��ѧ��Ӧ��
(1)ûʳ���Ậ�з��ǻ������ţ��������η�����ɫ��Ӧ����������īˮ����ѡB��
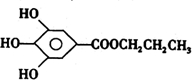
(2)ûʳ���Ậ���Ȼ������ţ������������������Ӧ������ûʳ�����������ṹ��ʽΪ
(3)�����������֪���Ჴ�������ɶ��ǻ��������붡������������Ӧ���ɣ������ʽΪC11H14O3��������ֻ�롪OH�͡�COOR����ȡ����ֱ������ʱ�����ڡ��䡢���������λ���칹��������(��R)�������ֽṹ(��CH2CH2CH2CH3��![]()
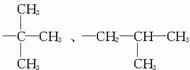 )������ͬ���칹�干��12�֡�
)������ͬ���칹�干��12�֡�
(4)�Ჴ������������������Һ���ȣ��ɷ�������ˮ�ⷴӦ����Ҫע����ǻ�Ҳ��������������Һ�����кͷ�Ӧ��
�𰸣�(1)B
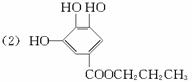
(3)C11H14O3 12
![]()
![]()



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

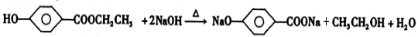
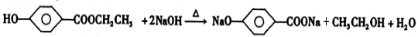
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
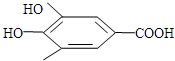
 300����ǰ��������ѧ�Ҳ����������������ûʳ���ᣨ�ṹʽ��ͼ��ʾ������ɫ��Ӧ�����ɴ˷���������īˮ������������īˮ��صĻ��ſ����ǣ�������
300����ǰ��������ѧ�Ҳ����������������ûʳ���ᣨ�ṹʽ��ͼ��ʾ������ɫ��Ӧ�����ɴ˷���������īˮ������������īˮ��صĻ��ſ����ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

 +CH3CH2OH+3CH3COONa
+CH3CH2OH+3CH3COONa +CH3CH2OH+3CH3COONa
+CH3CH2OH+3CH3COONa

| Ũ���� |
| 170�� |
| Ũ���� |
| 170�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
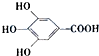
300����ǰ��������ѧ�Ҳ����������������ûʳ�������ɫ��Ӧ�����ɴ˷���������īˮ��ûʳ����ĽṹʽΪ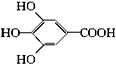 ��
��
��1����ûʳ��������īˮ��Ҫ������____________���������ʣ�����ţ���
A.�� B.�� C.��֬ D.����
��2��ûʳ����������п��������ã���Ŀǰ�㷺Ӧ�õ�ʳƷ���Ӽ�����ṹ��ʽΪ________________________________��
��3���Ჴ�����Ƕ��ǻ��������봼�γɵ��������ǹ�������ʹ�õ�ʳƷ���������Ჴ�����ķ���ʽΪ___________________���䱽��ֻ�롪OH�͡�COOR����ȡ����ֱ��������ͬ���칹����________________�֡�
��4��д���Ჴ������������������Һ���ȷ�Ӧ�Ļ�ѧ����ʽ��____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
300����ǰ��������ѧ�Ҳ����������������ûʳ�������ɫ��Ӧ�����ɴ˷���������īˮ��ûʳ����ĽṹʽΪ��
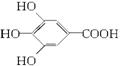
��1����ûʳ��������īˮ��Ҫ������___________���������ʣ�����ţ���
A.�� B.�� C.��֬ D.����
��2��ûʳ����������п��������ã���Ŀǰ�㷺Ӧ�õ�ʳƷ���Ӽ�����ṹ��ʽΪ______________________________________________________��
��3���Ჴ�����Ƕ��ǻ��������봼�γɵ��������ǹ�������ʹ�õ�ʳƷ���������Ჴ�����ķ���ʽΪ_____________________________���䱽��ֻ�롪OH�͡�COOR����ȡ����ֱ��������ͬ���칹����_____________�֡�
��4��д���Ჴ������������������Һ���ȷ�Ӧ�Ļ�ѧ����ʽ��______________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com