
���� ��1����8.0g CH4��ȫȼ������Һ��ˮ�ų�����1mol����ȼ�շų���������
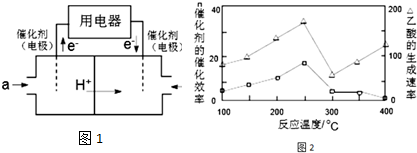
��2����ͼ��֪��ͨ������a��һ�˷���������Ӧ����Ӧͨ����飬�ü�Ϊ������ͨ��bΪ��������õ��ӣ����������½������������ˮ��
��3���ٷ���ͼ�������ᷴӦ��������������ѡ��
��CuAlO2�ܽ���ϡ���������������β��ų�NO���壬���ɵ���Ϊ������������ͭ����Ӧ����ˮ���ɣ���ƽ��д���ӷ���ʽ��
��4�����ݵ���ת���غ���㣮
��� �⣺��1����֪8.0g CH4��ȫȼ������Һ��ˮ�ų�444.8kJ��������1mol��16gȼ�շų�������Ϊ$\frac{16g}{8g}$��444.8kJ=889.6kJ����Ӧ���ȣ���ʡ�H=-889.6kJ/mol��
�ʴ�Ϊ��-889.6��
��2����ͼ��֪��ͨ������a��һ�˷���������Ӧ����Ӧͨ����飬�ü�Ϊ������ͨ��bΪ��������õ��ӣ����������½������������ˮ�������缫��ӦʽΪ��O2+4e-+4 H+=2H2O��
������ O2+4e-+4H+=2H2O��
��3���ٴ�ͼ���֪��250��ʱ���ᷴӦ����������ԣ���ѡ��250�棻
�ʴ�Ϊ��250�棻
��CuAlO2�ܽ���ϡ���������������β��ų�NO���壬���ɵ���Ϊ������������ͭ����Ӧ����ˮ���ɣ���Ӧ���ӷ���ʽΪ��3 CuAlO2+16 H++NO3-=NO��+3 Al3++3Cu2++8H2O��
�ʴ�Ϊ��3 CuAlO2+16 H++NO3-=NO��+3 Al3++3Cu2++8H2O��
��4�����ݵ�ʧ����ת���غ㣬��8.96L��[4-��-4��]=22.4L��2x�����x=1.6��
�ʴ�Ϊ��1.6��
���� �����漰��Ӧ�ȼ��㡢ԭ��ء���Ӧ�������ơ����ӷ���ʽ��д��������ԭ��Ӧ����ȣ��Ƕ�ѧ���ۺ������Ŀ��飬ע���H�з��ţ��״����Ѷ��еȣ�


 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ����� | �¶ȣ��棩 | ��ʼCCl4Ũ�ȣ�mol•L-1�� | ��ʼH2Ũ�ȣ�mol•L-1�� | CCl4��ƽ��ת���� |
| 1 | 110 | 1 | 1 | 50% |
| 2 | 100 | 1 | 1 | X |
| 3 | 110 | 0.8 | Y | 60% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com