
һ��������Һ̬������XY2���ڱ�״���µ�һ��������O2��ǡ����ȫȼ�գ���Ӧ����ʽΪ��XY2(l)��3O2(g)===XO2(g)��2YO2(g)����ȴ���ڱ�״���²��������������672 mL���ܶ���2.56 g/L����
(1)��ӦǰO2�������________________��
(2)������XY2��Ħ��������________________��
(3)��XY2������X��Y��Ԫ�ص���������3��16����X��Y��Ԫ�طֱ�Ϊ________��__________(дԪ�ط���)����д�� Y�����ӽṹʾ��ͼΪ ��
(4)��֪XԪ����aX��bX��cX����ԭ�ӣ�YԪ����eY��f Y ����ԭ�ӣ��������ܹ��γ� ��XY2���ӡ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ��������Һ̬������XY2����һ��������O2��ǡ����ȫȼ�գ���ѧ����ʽΪXY2(l)+3O2(g)=XO2(g)+2YO2(g)����ȴ���ڱ�״���²��������������672 mL,�ܶ���2.56 g/L��
(1)��ӦǰO2������� mL��
(2)������XY2��Ħ�������� g/mol��
(3)��XY2������X��Y��Ԫ�ص���������3��16����X��Y��Ԫ�طֱ�Ϊ �� ��(дԪ�ط���)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ��������Һ̬������XY2����һ������O2��ǡ����ȫȼ�գ���Ӧ����ʽΪ��XY2��Һ��+3O2(��)=XO2������+2YO2����������ȴ���ڱ�״���²��������������672mL���ܶ���2.56g/L����
��1����ӦǰO2����� �� ��2��������XY2��Ħ�������� ��
��3����XY2������X��Y����Ԫ�ص���������3��16 ����X��Y����Ԫ�طֱ�Ϊ
�� ����дԪ�ط��ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�����и�����һ���¿���ѧ�Ծ��������棩 ���ͣ������
�����12�֣�
��Ԫ�ص��⻯����������ڹ�ҵ�������������ж��й㷺Ӧ�á��ش��������⣺
(1)��Ԫ��ԭ�ӵ�L�������Ϊ ��
(2) NH3��NaClO��Ӧ�ɵõ���(N2H4)���÷�Ӧ�Ļ�ѧ����ʽΪ ��
(3)�¿���Ϊ�����������ȼ�ϣ���������N2O4��Ӧ����N2��ˮ������
��֪����N2(g)+2O2(g) = N2O4(l) ��H1=-19.5kJ∙mol��1
��N2H4(l) + O2 (g)=N2(g)+2H2O(g) ��H2 =-534.2 kJ��mol��1
д���º�N2O4��Ӧ���Ȼ�ѧ����ʽ ��
(4)��һ����ȼ�ϵ����һ�ּ��Ե�أ��õ�طŵ�ʱ�������ķ�ӦʽΪ ��
��һ��������Һ̬������ �ڱ�״���µ�һ��������
�ڱ�״���µ�һ�������� ��ǡ����ȫȼ��,��Ӧ����ʽΪ:
��ǡ����ȫȼ��,��Ӧ����ʽΪ: 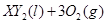 ===
===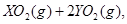 ��ȴ��,�ڱ�״���²��������������672 mL,�ܶ���2.56
��ȴ��,�ڱ�״���²��������������672 mL,�ܶ���2.56  ��:
��:
(1)��Ӧǰ �������
��
(2)������
�������
��
(2)������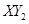 ��Ħ��������
��
��Ħ��������
��
(3)��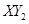 ������X��Y��Ԫ�ص���������3��16,��X��Y��Ԫ�طֱ�Ϊ
��
(дԪ�ط���)��
������X��Y��Ԫ�ص���������3��16,��X��Y��Ԫ�طֱ�Ϊ
��
(дԪ�ط���)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com