
�л���Aֻ����C��H��O����Ԫ�أ��������л��ϳɵ��м��塣16�� 8g���л��ᆳȼ������44.0g CO2��14��4g H2O������ͼ��������Է�������Ϊ84���������������A�����к���O��H����λ�ڷ��Ӷ˵�C��C�����˴Ź��������������壬�����Ϊ6��1��1��
��1��A�ķ���ʽ��____��A�Ľṹ��ʽ�� ��
��2�����������У�һ����������A������Ӧ���ǡ�����
A��H2 B��Na C��KMnO4 D��Br2
��3���л���B��A��ͬ���칹�壬1mol B����1mol Br2�ӳɡ����л�������̼ԭ����ͬһ��ƽ�棬û��˳���칹����B�к��������ŵ������� ��B�Ľṹ��ʽ��____ ��
��4��B����ͨ�����б仯�õ���Ԫ��״������F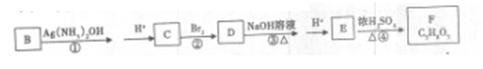
��д����Ӧ�ۻ�ѧ����ʽ ��
����ת����ϵ�Т١��ڡ��ܵķ�Ӧ�����ǣ�____ �� �� ��
 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ�꽭��ʡ��������ѧ�߶���һ���¿���ѧ�Ծ� ���ͣ�������
��8�֣�ij�л���Aֻ����C��H��O����Ԫ �أ�������
�أ������� �л��ϳɵ��м��塣�ֽ�8.4 g���л�����13.44L������£�O2�����ȼ�պ����������壨���л����ͨ��������CuSO4���壬���ָù�������7.2g��Ȼ���ٽ���ʣ������ͨ�������ij���ʯ��ˮ��ʯ��ˮ����17.6 g������ͼ��������Է�������Ϊ84���������������A�����к���O��H����λ�ڷ��Ӷ˵�C��C�����˴Ź��������������壬�����Ϊ6:1:1��
�л��ϳɵ��м��塣�ֽ�8.4 g���л�����13.44L������£�O2�����ȼ�պ����������壨���л����ͨ��������CuSO4���壬���ָù�������7.2g��Ȼ���ٽ���ʣ������ͨ�������ij���ʯ��ˮ��ʯ��ˮ����17.6 g������ͼ��������Է�������Ϊ84���������������A�����к���O��H����λ�ڷ��Ӷ˵�C��C�����˴Ź��������������壬�����Ϊ6:1:1��
��1��ͨ������ȷ�����л���A�ķ���ʽ����д��A�Ľṹ��ʽ��Ҫ�м�����̣���
��2���л���B��A��ͬ�� �칹�壬1 mol B����1mol Br2�ӳɡ����л�������̼ԭ����ͬһ��ƽ�棬û��˳���칹������д��B�Ľṹ��ʽ�ǡ�
�칹�壬1 mol B����1mol Br2�ӳɡ����л�������̼ԭ����ͬһ��ƽ�棬û��˳���칹������д��B�Ľṹ��ʽ�ǡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
����13�֣� �л���Aֻ����C��H��O����Ԫ�أ��������л��ϳɵ��м��塣16.8g���л�����ȫȼ������44.0g CO2��14.4g H2O������ͼ��������Է�������Ϊ84���������������A�����к���O��H����λ�ڷ��Ӷ˵�̼̼�������˴Ź��������������壬�����Ϊ6�s1�s1��
��1��A�ķ���ʽ�� �� ��2��A�Ľṹ��ʽ�� ��
��3�����������У�һ����������A������Ӧ���� (����ĸ)��
A��H2 B��Na C��KMnO4 D��Br2
��4���л���B��A��ͬ���칹�壬1mol B����1mol Br2�ӳɡ����л�������̼ԭ����ͬһ��ƽ�棬û��˳���칹������B�Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com