
(15��)ijУ��һ��ѧ�о���ѧϰС����ճ������е�������;�����˵��飬�˽�����ɹ㷺��Ӧ���������Ư�ס�ˮ���ɱ���������ȡ�
(1)������������Ư�ס�������ԭ������Ϊ����ˮ�����γɾ���______�ԵĴ����ᣬ��ط�Ӧ�Ļ�ѧ����ʽΪ____________________________��
(2)�ڵ���ij������Ӿ���ļ���ˮ�������ʱ��С���Ա�˽������Ӿ��ÿ��һ����8��00��ˮ��Ȼ��ͨ������������ͨ����Ӿ����ˮ�ĺ�����������0.5 mg��L��1��1.0 mg��L��1֮��ʱ��Ч����á���ͼ�Ǹ�С��ⶨ��ÿ��19��00ʱ��Ӿ����ˮ�ĺ��������ļ���ʹ����Ӿ�ز���ȫ��______��
(3)����Ϊ�ļ�����������ȡ�����ǿ��______��˵��һ��������
__________________________(��Ҫ�ķ���ʽ������)��
(4)�ڶ���Ӿ��ˮ��ͨ����������ʱ������������й©ʱ��Ӧ�����ر������ޣ���Ӧ��ȡ�����Ծȷ���______��
A����ʪ���ë����ס�ڱ�����ʹ�
B���ý�ʪС�մ�����ˮ��ë����ס�ڱ�����ߴ�
C���ý�ʪŨ��ˮ��ë����ס�ڱ�����������ȫ��
D���ý�ʪʳ��ˮ��ë����ס�ڱ�˳��������ȫ��
(5)С����Ӿ��ͨ��ʹ��Ư��Һ(NaClO��Һ)����������������ˮ���Ծٳ�ʹ��Ư��Һ��������������һ������__________________���û�ѧ����ʽ˵����ҵ���������Ư��Һ��________________________________________________________________________��
(1)ǿ������Cl2��H2O===HCl��HClO
(2)��������������
(3)�����ġ�������������ǿ��HClO���ֽ⣬2HClO2HCl��O2�������º������½�����
(4)B
(5)NaClO���ȶ������ڴ�������䡡2NaOH��Cl2===NaCl��NaClO��H2O?
����:��������ˮ��HClO������Ϊ�������������Ŀ��
(1)����HClO��ǿ������ɱ��������(3)��ΪHClO���й����ֽ�����ʣ�2HClO2HCl��O2����ʹClO��Ũ�Ƚ��ͣ���Ч�Ƚ��ͣ�ɱ������Ч�������롣��������ÿ��ı仯�����ɼ����ġ������½��������ԣ��������ǿ�ҡ�(4)��Cl2�ܶȱȿ�������Ӧ��ߴ��ܣ�����Ϊ��������ˮʱ���ɵ�HCl��HClO�����ԣ���Ӧ���Լ��Ե��������գ����ǵ���ȫ�ԣ��ʿ��ý���С�մ�����ˮ��ë����ס�ڱǡ�(5)��ΪHClO�ļ��ⲻ�ȶ��ԣ����ѡ��������Һɱ��������Ч�������ұ��ڴ�������䡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��³��1����ʶ��ѧ��ѧ��ϰ ���ͣ�����
(15��)ijУ��һ��ѧ�о���ѧϰС����ճ������е�������;�����˵��飬�˽�����ɹ㷺��Ӧ���������Ư�ס�ˮ���ɱ���������ȡ�
(1)������������Ư�ס�������ԭ������Ϊ����ˮ�����γɾ���______�ԵĴ����ᣬ��ط�Ӧ�Ļ�ѧ����ʽΪ____________________________��

(2)�ڵ���ij������Ӿ���ļ���ˮ�������ʱ��С���Ա�˽������Ӿ��ÿ��һ����8��00��ˮ��Ȼ��ͨ������������ͨ����Ӿ����ˮ�ĺ�����������0.5 mg��L��1��1.0 mg��L��1֮��ʱ��Ч����á���ͼ�Ǹ�С��ⶨ��ÿ��19��00ʱ��Ӿ����ˮ�ĺ��������ļ���ʹ����Ӿ�ز���ȫ��______��
(3)����Ϊ�ļ�����������ȡ�����ǿ��______��˵��һ��������
__________________________(��Ҫ�ķ���ʽ������)��
(4)�ڶ���Ӿ��ˮ��ͨ����������ʱ������������й©ʱ��Ӧ�����ر������ޣ���Ӧ��ȡ�����Ծȷ���______��
A����ʪ���ë����ס�ڱ�����ʹ�
B���ý�ʪС�մ�����ˮ��ë����ס�ڱ�����ߴ�
C���ý�ʪŨ��ˮ��ë����ס�ڱ�����������ȫ��
D���ý�ʪʳ��ˮ��ë����ס�ڱ�˳��������ȫ��
(5)С����Ӿ��ͨ��ʹ��Ư��Һ(NaClO��Һ)����������������ˮ���Ծٳ�ʹ��Ư��Һ��������������һ������__________________���û�ѧ����ʽ˵����ҵ���������Ư��Һ��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�갲��ʡ�����и����ڶ��ε��в��ԣ����ۣ���ѧ���� ���ͣ�ʵ����
(15��) ijУ��ѧ��ȤС��Ϊ��̽����������������������ͭ���ʣ���ijŨ�����ᷴӦ�����������̽�����
̽��һ
��1����������Ͷ��ijŨ�������У�ijͬѧ�۲��ʵ������ʱ���֣���Ӧ����һ��ʱ���Ӧ��ʼ�ӿ졣�������Ӧ�ӿ�Ŀ���ԭ��
_______________________________________________________________________��
��2������ȡ������10 g����ijŨ�������У���ַ�Ӧ��õ���ҺX���ռ�������Y��Ϊ��̽����ҺX����Ԫ�صļ�̬,ͬѧ���������ʵ�飺��ҩƷ��������0.1 mol��L-1 KSCN��Һ��0.1 mol��L-1 KI��Һ��0.2 mol��L-1���Ը��������Һ����ˮ�ȣ��Թܺ͵ι�
������Ƽ�ʵ�飬̽�����������Ƿ���ȷ����д����ʵ�鱨�棺
|
ʵ�鲽�� |
���� |
���� |
���ӷ���ʽ |
|
��һ�� |
ȡ2-3 mL��Һװ���Թܣ����Թ��м��뼸��KSCN��Һ�� |
|
|
|
�ڶ��� |
|
����Һ��ɫ��ȥ������Һ����Fe2+�� �������Ա仯����Fe3+�� |
|
̽���� Ϊ��̽������Y�ijɷ֣�ͬѧ�����������װ�ã�������NO2ת��ΪN2O4��

(3)װ���ҵ����ã� ��
��4��װ�ñ��ռ����������ͨ�����ݹ���������к���ɫ�������ɣ��ܷ�ȷ��
����Y�к�NO�� ˵�����ɡ�
________________________________________________________________________��
(5) ͬѧ��Ϊ��̽������Y����ɣ���224mL����Yͨ��������NaOH��Һ�У����屻��ȫ���գ�������Һ����0.15 mol��L-1����KMnO4��Һ�ζ�������20 mLKMnO4��Һ��
������Y��NO��NO2�������Ϊ ��
����֪2NO2+2NaOH��NaNO3+NaNO2+H2O��NO2+NO+2NaOH��2NaNO2+H2O��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)ijУ��һ��ѧ�о���ѧϰС����ճ������е�������;�����˵��飬�˽�����ɹ㷺��Ӧ���������Ư�ס�ˮ���ɱ���������ȡ�
��������������Ư�ס�������ԭ������������ˮ�����γɾ��� �ԵĴ����ᡣ
 ��ط�Ӧ�����ӷ���ʽΪ ��
��ط�Ӧ�����ӷ���ʽΪ ��
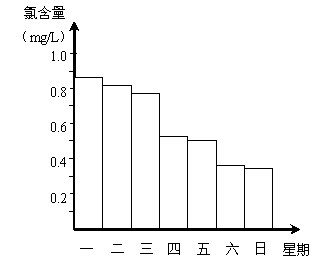
���ڵ���ij������Ӿ���ļ���ˮ�������ʱ��С���Ա�˽������Ӿ��ÿ��һ����8��00��ˮ��Ȼ��ͨ���������������Ⱥ���Ϊ0.9mg/L��(�Ⱥ���ָÿ��ˮ�к�Cl2��������ͨ����Ӿ��ˮ���Ⱥ���������0.5 mg/L��1.0mg/L֮��ʱ��Ч�����)��ͼ�Ǹ�С��ⶨ��ijһ��ÿ��19��00ʱ��Ӿ����ˮ���Ⱥ��������ļ���ʹ����Ӿ�ز����� ��
�Ǹ�����ͼ������Ϊ��һ�����ļ�����������ȡ�����ǿ�� ��
˵��һ�������� ��
���ڶ���Ӿ��ˮ��ͨ����������ʱ������������й©��Ӧ�����ر������ޣ���Ӧ��ȡ�����Ծȷ��� ����ѡ����ţ�
A����ʪ���ë����ס�ڱ�����ʹ�
B���ý�ʪС�մ�����ˮ��ë����ס�ڱ�����ߴ�
C���ý�ʪŨ��ˮ��ë����ס�ڱ�����ǰ������ȫ��
D���ý�ʪʳ��ˮ��ë����ס�ڱ�˳��������ȫ��
��С����Ӿ��ͨ��ʹ��Ư��Һ��NaClO��Һ������������������ˮ���Ծٳ�ʹ��Ư��Һ����������һ������ ��
����д����֪��������������ˮ������������������(����д����) �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)ijУ��һ��ѧ�о���ѧϰС����ճ������е�������;�����˵��飬�˽��
���ɹ㷺��Ӧ���������Ư�ס�ˮ���ɱ���������ȡ�
��������������Ư�ס�������ԭ������������ˮ�����γɾ��� �ԵĴ����ᡣ
��ط�Ӧ�����ӷ���ʽΪ ��
 ���ڵ���ij������Ӿ���ļ���ˮ�������ʱ��
���ڵ���ij������Ӿ���ļ���ˮ�������ʱ��
С���Ա�˽������Ӿ��ÿ��һ����8��00
��ˮ��Ȼ��ͨ���������������Ⱥ���Ϊ0.9mg/L��
(�Ⱥ���ָÿ��ˮ�к�Cl2��������ͨ����Ӿ��ˮ����
����������0.5 mg/L��1.0mg/L֮��ʱ��Ч�����)
��ͼ�Ǹ�С��ⶨ��ijһ��ÿ��19��00ʱ��Ӿ��
��ˮ���Ⱥ��������ļ���ʹ����Ӿ�ز����� ��
�Ǹ�����ͼ������Ϊ��һ�����ļ�����������ȡ�����ǿ�� ��
˵��һ�������� ��
���ڶ���Ӿ��ˮ��ͨ����������ʱ������������й©��Ӧ�����ر������ޣ���Ӧ��ȡ����
�Ծȷ��� ����ѡ����ţ�
A����ʪ���ë����ס�ڱ�����ʹ�
B���ý�ʪС�մ�����ˮ��ë����ס�ڱ�����ߴ�
C���ý�ʪŨ��ˮ��ë����ס�ڱ�����ǰ������ȫ��
D���ý�ʪʳ��ˮ��ë����ס�ڱ�˳��������ȫ��
��С����Ӿ��ͨ��ʹ��Ư��Һ��NaClO��Һ������������������ˮ���Ծٳ�ʹ��Ư��Һ��
��������һ������ ��
����д����֪��������������ˮ������������������(����д����) �� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com