
 ������I����Ϊδ֪���ֵĽṹ��
������I����Ϊδ֪���ֵĽṹ��








| 2��12+2-13-1 |
| 2 |
 �����Ͽ�֪X�Ľṹ��ʽΪ��
�����Ͽ�֪X�Ľṹ��ʽΪ�� ����G�Ľṹ��ʽΪ��
����G�Ľṹ��ʽΪ�� ���ݴ˽��
���ݴ˽��| 2��12+2-13-1 |
| 2 |
 �����Ͽ�֪X�Ľṹ��ʽΪ��
�����Ͽ�֪X�Ľṹ��ʽΪ�� ����G�Ľṹ��ʽΪ��
����G�Ľṹ��ʽΪ�� ��
�� ������G�к����Ȼ��ʹ��ǻ����ʴ�Ϊ��HOOC-COOH���Ȼ������ǻ���
������G�к����Ȼ��ʹ��ǻ����ʴ�Ϊ��HOOC-COOH���Ȼ������ǻ���  ��E�ܷ����ӳɷ�Ӧ��ȡ����Ӧ��������Ӧ�����ܷ�����ȥ��Ӧ����ѡB��
��E�ܷ����ӳɷ�Ӧ��ȡ����Ӧ��������Ӧ�����ܷ�����ȥ��Ӧ����ѡB�� ��
�� ��
�� ��G��һ�������·�����Ӧ���ɷ������ΪC4H4O4���л�����л����ʹ������Ȼ�̼��Һ��ɫ����˵���������к���̼̼˫�������ݷ���ʽ֪��G������ȥ��Ӧ����Ӧ����ʽΪ��
��G��һ�������·�����Ӧ���ɷ������ΪC4H4O4���л�����л����ʹ������Ȼ�̼��Һ��ɫ����˵���������к���̼̼˫�������ݷ���ʽ֪��G������ȥ��Ӧ����Ӧ����ʽΪ�� ��
�� ��
�� ��
�� ��
�� ��G��ͬ���칹�壬a���ܷ���������Ӧ��˵������ȩ����b������NaHCO3��Ӧ˵�������Ȼ���c��1mol��������������Na��Ӧ�ų�1.5molH2��˵��������һ�����ǻ���������ṹ��ʽΪ
��G��ͬ���칹�壬a���ܷ���������Ӧ��˵������ȩ����b������NaHCO3��Ӧ˵�������Ȼ���c��1mol��������������Na��Ӧ�ų�1.5molH2��˵��������һ�����ǻ���������ṹ��ʽΪ ��
�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


 Z+H2O��д���÷�Ӧ�Ļ�ѧ����ʽ
Z+H2O��д���÷�Ӧ�Ļ�ѧ����ʽ



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �������������غ㶨�ɣ����жϳ���һ����Ӧ����Ϊ
�������������غ㶨�ɣ����жϳ���һ����Ӧ����Ϊ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij�л���X�ļ���ʽΪ��
ij�л���X�ļ���ʽΪ��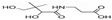 �������л���X��˵����ȷ���ǣ�������
�������л���X��˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢ڢ� | B���٢ۢ� | C���٢ڢ� | D���٢ڢۢܢ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com