
�������и��������������ݣ��ɷֱ�����䡰���ʵ����������������ʵ����ʵ���Ũ�ȡ������жϲ���⡣
��1����NA��ʾ�����ӵ���������ֵ����ij����������ҺV L�к���N��OH-������������Һ��______Ϊ______��
��2����֪ij����������Һ��Na����H2O�ĸ���֮��Ϊ1��a������������Һ��______Ϊ______��
��3����֪��״����1���ˮ���ܽ�500������Ȼ��⣬��������״�����Ȼ��ⱥ����Һ��______Ϊ______��
��4����֪��100 mL�Ȼ�����ˮ��Һ�����������գ��ɵõ���ɫ����b g��������ԭ�Ȼ�����Һ��______Ϊ______��
��1��NaOH�����ʵ���Ũ�� 
��2��NaOH���������� 
��3��HCl���������� 44.9%
��4��AlCl3�����ʵ���Ũ�� 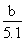 mol/L
mol/L
����������1����֪��Һ���ܶȣ�ֻ��������ʵ���Ũ�ȣ�c��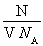 mol/L��
mol/L��
��2��NaOH��H2O�����ʵ���֮��Ϊ1��a���������ʵ�����������w�� ��
��
��3����֪��Һ���ܶȣ����ܼ������ʵ���Ũ�ȣ�����������������
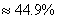 ��
��
��4����ɫ����ΪAl2O3��n��Al2O3����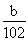 mol��n��AlCl3����
mol��n��AlCl3����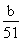 mol��c��AlCl3����
mol��c��AlCl3����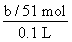 ��
�� mol/L��
mol/L��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 2-2���ӷ�Ӧ��ϰ���������棩 ���ͣ�ѡ����
�����������и���������ָ����Һ��һ���ܴ������������ ��
A.1.0 mol��L-1��KNO3��Һ��H+��Fe2+��Cl-��
B.���ȳʺ�ɫ����Һ�� ��Ba2+��
��Ba2+�� ��Cl-
��Cl-
C.pH=12����Һ��K+��Na+��CH3COO-��Br-
D.������Ӧ����������������Һ��Na+��K+��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 11-3���ʵ��Ʊ���ϰ���������棩 ���ͣ�ѡ����
�����й�ʵ�������������ȷ����( )
A.��50 mL��ʽ�ζ��ܿ�ȷ��ȡ25.00 mLKMnO4��Һ
B.����Ͳ��ȡ5.00 mL 1.00 mol��L-1������50 mL ����ƿ�У���ˮϡ�����̶ȣ�������0.100 mol��L-1����
C.�ñ���ȡ��ˮ�е��壬��Һʱ�л���ӷ�Һ©�����¶˷ų�
D.ʵ���ҳ������ˮ����μ��뱥��FeCl3��Һ���������ȵķ����Ʊ�������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 11-1��ѧʵ�������ͻ���������ϰ���������棩 ���ͣ�ѡ����
�������и�װ��ͼ�������У��������( )

A.װ�â�������ⱥ��ʳ��ˮ��c�缫������������ʹʪ��ĵ���KI��ֽ����
B.װ�âڿ������ռ�H2��NH3��Cl2��HCl��NO2
C.װ�â���XΪ�������������հ���������
D.װ�âܿ����ڸ���ռ������������ն���İ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 11-1��ѧʵ�������ͻ���������ϰ���������棩 ���ͣ�ѡ����
�߱������Ļ�ѧʵ�鼼���ǽ��п�ѧ̽���Ļ����ͱ�֤�������й�ʵ�������ȷ����( )

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 1-2���ʵ�����ʵ���е�Ӧ����ϰ���������棩 ���ͣ�ѡ����
����̼���ƾ��壨Na2CO3��10H2O��������0.5 mol/L��̼������Һ1 000 mL������������������ȷ����ģ��������������������Һ��Ũ��ƫ�ߵ��ǣ� ��
A.��ȡ̼���ƾ���100 g
B.����ʱ���ӿ̶���
C.��Һʱ���������ܽ�̼���ƾ�����ձ�û�н��г�ϴ
D.���ݺ�����ƿ���ȣ����÷���Һ����ڿ̶��ߣ������ּ�������ˮ���̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 1-1���ʵ��� ����Ħ�������ϰ���������棩 ���ͣ������
������������ȷ��Ԫ��X�����ԭ��������
��1�� 1.01��105 Pa,273 ��ʱ��̬����Xn���ܶ�Ϊd g/L����X�����ԭ������Ϊ_____��
��2����ͬ״���£�һ�������X����̬�⻯��HmX�������ǵ����NH3��2������X�����ԭ������Ϊ_____��
��3��a��Xԭ�ӵ�������Ϊb g����X�����ԭ�������ɱ�ʾΪ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��Ԫ��� �����·ǽ������仯������ϰ���������棩 ���ͣ�ѡ����
���б��к��ߵ������ڸ����������²�����ȫ�ܽ����( )
��1 mol Zn�뺬1 mol H2SO4��ϡ������Һ��Ӧ
��1 mol Cu�뺬2 mol H2SO4��Ũ������Һ����
��1 mol Cu�뺬4 mol HNO3��Ũ������Һ��Ӧ
��1 mol MnO2�뺬4 mol HCl��Ũ������Һ����
A.�٢� B.�٢� C.�ڢ� D.�ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��Ԫ��� ���������»�ѧ��Ӧ����������ƽ����������棩 ���ͣ������
(1)��ѧƽ�ⳣ��K��ʾ���淴Ӧ�Ľ��г̶ȣ�KֵԽ��ʾ���淴Ӧ���е�Խ��ȫ��Kֵ��С���¶ȵĹ�ϵ�ǣ��¶����ߣ�Kֵ (����һ������������һ����С��������������Ҳ���ܼ�С��)��
(2)��һ���Ϊ10 L�������У�ͨ��һ������CO��H2O����800��ʱ�������·�Ӧ��CO(g)+H2O(g) CO2(g)+H2(g)��H��0��CO��H2O�����ʵ���Ũ�ȱ仯��ͼ��ʾ����
CO2(g)+H2(g)��H��0��CO��H2O�����ʵ���Ũ�ȱ仯��ͼ��ʾ����

��0��4 minʱ���ƽ����Ӧ����v(CO)�� mol��L-1��min-1��
����800��ʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ��K�� (Ҫ��д������ʽ����ֵ)��CO��ת���ʣ� ��
����800��ʱ������Ӧ��ʼʱ��������CO��H2O��Ũ�ȷֱ�Ϊ0.20 mol��L-1��0.80 mol��L-1����ﵽƽ��ʱCOת��ΪCO2��ת������ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com