
��ͼ��ʾ��ͨ��5 min�缫5������������2.16 g���ش�
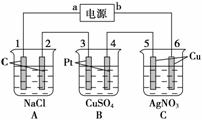
(1)��Դ��a��________����C����________�ء�
A�������缫��ӦʽΪ__________________�������缫��ӦʽΪ__________________��C�������缫��Ӧʽ__________________�������缫��ӦʽΪ
________________________________________________________________________��
(2)���B���й��ռ���224 mL����(��״��)������Һ���Ϊ200 mL(�����������Һ�������)����ͨ��ǰ��Һ��Cu2�������ʵ���Ũ��Ϊ
________________________________________________________________________��
(3)���A����ҺҲ��200 mL(����������Һ�������)����ͨ�����Һ��pHΪ________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ʺ���ij��ԭ�Ӿ������ (����)��
A���۵�1 070 �棬������ˮ��ˮ��Һ����
B���۵�10.32 �棬Һ̬�����磬ˮ��Һ����
C��������CS2���۵�112 �棬�е�444.6 ��
D���۵�3 550 �棬��Ӳ��������ˮ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�������ݣ�
| ���� | �۵�(��) | �е�(��) | �ܶ�(g·cm��3) |
| �Ҵ� | ��117.0 | 78.0 | 0.79 |
| ���� | 16.6 | 117.9 | 1.05 |
| �������� | ��83.6 | 77.5 | 0.90 |
| Ũ����(98%) | — | 338.0 | 1.84 |
ѧ����ʵ������ȡ������������Ҫ�������£�
����30 mL�Ĵ��Թ�A�а������1��4��4�ı�������Ũ���ᡢ�Ҵ�������Ļ����Һ��
�ڰ���ͼ���Ӻ�װ��(װ������������)����С����ȵؼ���װ�л����Һ�Ĵ��Թ�5��10
min��
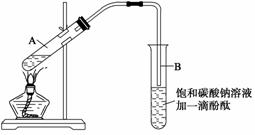
�۴��Թ�B�ռ���һ�����IJ����ֹͣ���ȣ���ȥ�Թ�B��������Ȼ���ô��ֲ㣻
�ܷ�������������㡢ϴ�ӡ����
�������ĿҪ��ش��������⣺
(1)���Ƹû����Һ����Ҫ��������Ϊ
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
д����ȡ���������Ļ�ѧ����ʽ��
________________________________________________________________________
________________________________________________________________________��
(2)����ʵ���б���̼������Һ��������(����
ĸ)________________________________________________________________________��
A���к�������Ҵ�
B���к����Ტ���ղ����Ҵ�
C�����������ڱ���̼������Һ�е��ܽ�ȱ���ˮ�и�С�������ڷֲ�����
D�������������ɣ���������
(3)���������ҪС����ȼ��Ȳ���������Ҫ������
________________________________________________________________________
________________________________________________________________________��
(4)���������������Ϊ�˸�������������ѡ�õĸ����Ϊ(����ĸ)______��
A��P2O5 B����ˮNa2SO4
C����ʯ�� D��NaOH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
2008��5��17���½�����̨(�������)�������ҹ���������̩�����������㡣���־�������������ء����������������½�����̩���ѳ�Ϊ�ҹ���8�����Ҵ����������㡣���㰲װ�ļ���豸�ɼ���ճ�����������������һ����������Ⱦ�NO��������Ⱦ����������������������������������NO����������������֯�д��ڣ���������Ѫ�ܡ����ߡ���ǿ����Ĺ��ܣ�����Ϊ��ǰ������ѧ���о��ȵ㣬NO�౻��Ϊ�����Ƿ��ӡ�����ش��������⣺
(1)NO�Ի�����Σ������________(����)��
A���ƻ�������
B����������ʹһЩ��������
C���������
D��������Ѫ�쵰���
(2)�ں�Cu����ø�Ļ���ģ������������(NO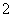 )��ת��ΪNO��д��Cu���������������������ˮ��Һ�з�Ӧ�����ӷ���ʽ��_________________________________________��
)��ת��ΪNO��д��Cu���������������������ˮ��Һ�з�Ӧ�����ӷ���ʽ��_________________________________________��
(3)����ɫ���ˡ���2008�걱�����˻������֮һ��Ϊӭ�Ӱ��ˣ����ٿ�����Ⱦ������Ϊ������װ�ˡ���Ч�������������ɽ�β���е�һ����̼��һ������ת��Ϊ�������ѭ������������壬��������(����)
A��������̼�͵��� B��������̼�Ͷ�������
C��������̼������ D��������̼������
(4)������(��CCl2F2)���ڹ�������·ֽ⣬������ԭ�ӣ���ԭ�ӻ�Գ�����������õ��ƻ�����(�����ķ���ʽΪO3)���йط�Ӧ���£�
O3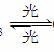 O2��O��Cl��O3ClO��O2��ClO��OCl��O2���ܷ�Ӧ��2O3===3O2
O2��O��Cl��O3ClO��O2��ClO��OCl��O2���ܷ�Ӧ��2O3===3O2
������������������Ĺ����У�Cl��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ħ��������˾�з���һ���ɼ״��������Լ�ǿ�����������Һ�������ֻ���أ������ɴ�����ʹ�õ������﮵�ص�ʮ����������ʹ��һ���²ų�һ�ε磬���ط�ӦʽΪ2CH3OH��3O2��4OH�� 2CO
2CO ��6H2O���������й�˵���������(����)
��6H2O���������й�˵���������(����)
A���ŵ�ʱCH3OH���뷴Ӧ�ĵ缫Ϊ����
B�����ʱ�������Һ��pH������
C���ŵ�ʱ�����ĵ缫��ӦʽΪCH3OH��6e����8OH��===CO ��6H2O
��6H2O
D�����ʱÿ����1 mol CH3OHת��6 mol����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NO2װ����������ܱ������У�����Ӧ2NO2(g)N2O4(g)�ﵽƽ��ı�����һ������������������ȷ���� (����)��
A�������¶ȣ�������ɫ�����˷�ӦΪ���ȷ�Ӧ
B������ѹ�����������ƽ�������ƶ������������ɫ��dz
C������ѹ������������������Сһ�룬ѹǿ����С��ԭ��������
D�����º���ʱ������������壬ѹǿ����ƽ�������ƶ�������������ɫ��dz
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�������о�����CO2�������״�ȼ�ϵķ������÷����Ļ�ѧ����ʽ�ǣ�
CO2(g)��3H2(g)CH3OH(g)��H2O(g) ��H����49.0 kJ/mol
�ֽ�6 mol CO2��8 mol H2����һ�ݻ�Ϊ2 L���ܱ������У����H2�����ʵ�����ʱ��仯��ͼ��ʵ����ʾ(ͼ����ĸ�������ڵ����Ա�ʾ��Ӧ������)��
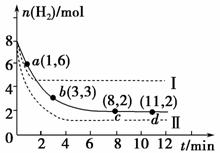
�ش��������⣺
(1)��ͼ������������ʱ����ڷ�Ӧ����������______(�����)��
a��0��1 min�� B��1��3 min
c��3��8 min�� D��8��11 min
(2)���ı�ijһ�����ٽ���ʵ�飬���H2�����ʵ�����ʱ��ı仯��ͼ��������ʾ����ʵ����ȣ����ߢ�ı������������________________�����ߢ�ı������������________________��
(3)���б����ܱ�ʾ�÷�Ӧ�Ѵ�ƽ�����________(�����)��
a��������ѹǿ���ٸı�
b��������������ܶȲ��ٸı�
c�������������ƽ��Ħ���������ٸı�
d�������ڸ����ʵ����ʵ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��pH��3��H2SO4��pH��12��NaOH��Һ��ϣ��������Һ��pH��10ʱ��ǿ���ǿ������֮��Ϊ(����)
A��1��9 B��9��1 C��10��1 D��1��10
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
)�ϳɰ����յ�һ����Ҫ������ͭϴ����Ŀ������ͭҺ[���������ͭ��I������ˮ]���������������в�����CO��CO2�����塣ͭҺ����CO�ķ�Ӧ�Ƿ��ȷ�Ӧ���䷴Ӧ����ʽΪ�� Cu(NH3)2Ac��CO��NH3 [Cu(NH3)3CO]Ac �����������գ�
[Cu(NH3)3CO]Ac �����������գ�
(1)���Ҫ���������Ӧ�ķ�Ӧ���ʣ����Բ�ȡ�Ĵ�ʩ�� ����ѡ���ţ�
a.��ѹ b.����NH3��Ũ�� c.���� d.��ʱ���߲���
(2)ͭҺ�еİ������ն�����̼��д���÷�Ӧ�Ļ�ѧ����ʽ_____________________
(3)ͭҺ�����Ԫ���У�������Ԫ��ԭ�Ӱ뾶�Ӵ�С������˳��Ϊ__________________��ͨ���Ƚ�_____________________________���жϵ��������ַǽ���Ԫ�صķǽ�����ǿ����
(4)��֪CS2��CO2���ӽṹ���ƣ�CS2�ĵ���ʽ��_________________��CS2�۵����CO2����ԭ���� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com