
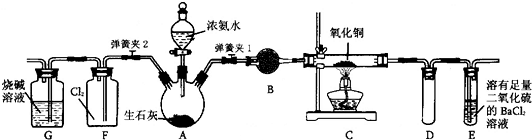
 Cu+N2��+H2O
Cu+N2��+H2O Cu+N2��+H2O
Cu+N2��+H2O


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
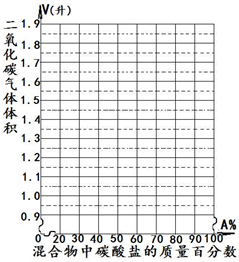
| ������̼������������� | 0.93 | 1.12 | 1.31 | 1.49 | 1.68 |
| ̼���ε������ٷ�����A%�� | 50 | 60 | 70 | 80 | 90 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
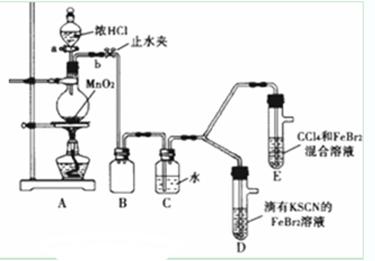
| ʵ����� | ʵ������ | ���� |
| ����a����Բ����ƿ�е�������Ũ���Ȼ��رջ���a����ȼ�ƾ��� | Dװ���У���Һ��� Eװ���У�ˮ����Һ�����CCl4�������Ա仯 | Cl2��Br2��Fe3+����������ǿ������˳��Ϊ�� ______________________ |
| ��SCN��2������±�ص������ƣ������ԣ�Cl2����SCN��2�� ��Cl2��Br2��Ӧ����BrCl�����ʺ�ɫ���Դ���ɫ�����е�Ϊ5�棬��ˮ����ˮ�ⷴӦ�� ��AgClO��AgBrO��������ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ������ | ʵ��Ŀ�Ļ�ʵ����� |
| A | ��ʢ��2 mL 0.1 mol/L AgNO3��Һ���Թ��еμ�5��0.1 mol/L NaCl��Һ���а�ɫ�������ɣ��������еμ�5��0.1 mol/L KI��Һ | ˵��һ�ֳ�����ת��Ϊ�ܽ�ȸ�С�ij��� |
| B | ��1 mL 20% ��������Һ�м���3��5��ϡ���ᣬˮԡ����5 min����ȴ���ټ�������Cu(OH)2����Һ������ | ֤�������ܷ���ˮ�ⷴӦ |
| C | ˮԡ����Ũ���ᡢŨ����ͱ��Ļ�����ֱ�������Һ��õ��Ĵֲ�Ʒ | �Ʊ��������� |
| D | ������,�ֱ���2֧�Թ��м�����ͬ�������ͬŨ�ȵ�Na2S2O3��Һ,�ٷֱ����������ͬŨ�ȵ�ϡ���� | �о�Ũ�ȶԷ�Ӧ���ʵ�Ӱ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ����� | ʵ������ | ���� |
| A | ij������Һ����AgNO3��Һ | �а�ɫ���� | �����β�һ����NaCl |
| B | Ũ������NaCl�����ϼ��� | ��������� | ��������Ա�HClǿ |
| C | ij��ɫ����ͨ����ˮ�� | ��ˮ��ɫ | ������һ����C2H4 |
| D | ��Ũ�Ⱦ�Ϊ0.1mol��L��1��NaCl��NaI�����Һ�еμ�������Pb(NO3)2��Һ | ���ֻ�ɫ���� ��PbI2�� | KSP��PbI2����KSP��PbCl2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
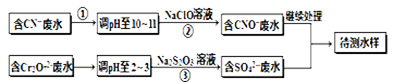
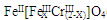 (��������,�������ֱ�ʾԪ�ؼ�̬)�ij���������1mol Cr2O72-�������a mol FeSO4?7H2O�����н�����ȷ���� ��
(��������,�������ֱ�ʾԪ�ؼ�̬)�ij���������1mol Cr2O72-�������a mol FeSO4?7H2O�����н�����ȷ���� ��| A��x ="0.5" ,a =8 | B��x ="0.5" ,a = 10 | C��x =" 1.5" ,a =8 | D��x =" 1.5" ,a = 10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
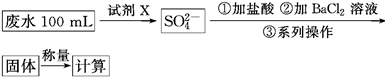
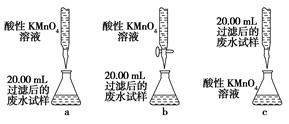
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
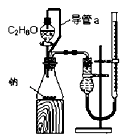 | 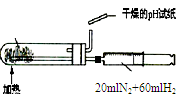 | 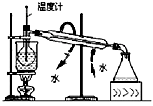 | 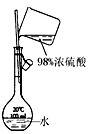 |
| A.�ⶨ�Ҵ����ӽṹ | B.�ϳɰ������鰱�����ɲ�����CCl4 | C.���CCl4��Һ�з���I2 | D.����ϡ������Һ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com