
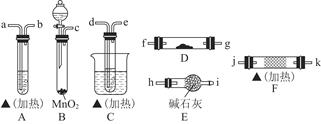
 2CH3CHO��2H2O���÷�ӦΪ���ȷ�Ӧ����Ӧ���̷ų���������ά�ַ�Ӧ��������
2CH3CHO��2H2O���÷�ӦΪ���ȷ�Ӧ����Ӧ���̷ų���������ά�ַ�Ӧ��������


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
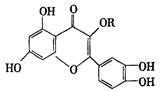
| A�����ӽṹ�ж������ǻ� |
| B��������NaOH��Һ�����кͷ�Ӧ |
| C����FeCl3��Һ��Ӧ����ɫ |
| D�������¶�����ɫҺ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
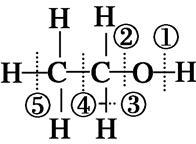
| A���ǻ�������������ͬ�Ļ�ѧʽ�͵���ʽ |
| B���Ҵ��Ĺ������ǡ�OH���Ҵ��Ǻ���OH�Ļ����� |
| C�������£�1 mol�Ҵ�����������Na��Ӧ����11.2 L H2 |
| D����֪�Ҵ��Ľṹʽ��ͼ��ʾ�����Ҵ�������ʱ���ѵĻ�ѧ��Ϊ�ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
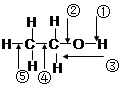
| A���ͽ���������ʱ�����ڶ��� |
| B����Ũ���Ṳ����170 ��ʱ�����ٺ͢ݶ��� |
| C�������ᡢŨ���Ṳ��ʱ�����ٶ��� |
| D����ͭ���º�������Ӧʱ�����ٺ͢ڶ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
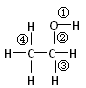
| A��������Ʒ�Ӧʱ���ٶ��� | B����ȥ��Ӧʱ���ڡ��۶��� |
| C����HBr��Ӧʱ�ڶ��� | D����������ȩʱ�ڡ��ܶ��� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com