


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
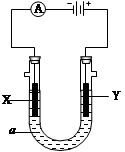
| A����ⱥ��ʳ��ˮ���ռ�������ӽ���Ĥ����������ֹ������ͨ�� |
| B�������϶�пʱ��п�����������ҵ��Һ����п��Ũ���Dz���� |
| C���ö��Ե缫���Na2SO4��Һ������������������ʵ���֮��Ϊ1: 2 |
| D���ö��Ե缫��ⱥ��NaCl��Һ������1 mol����ת�ƣ�������1 molNaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
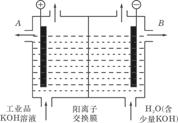
| A��4.48L | B��5.6L | C��6.72L | D��11.2L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
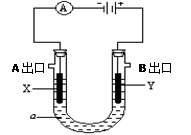
| A��b<a<7 |
| B�������缫��ӦʽΪ��Cu2++2e-="Cu" |
| C������Һ�м���9.8x(10-b-10-a)gCu(OH)2��ʹ��Һ�ָ������ǰ��Ũ�� |
| D������������������O2�������(��״����)Ϊ1.12x(10-b-10-a)L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��35.5:108 | B��16:207 | C��108:35.5 | D�� 8:1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��0.2mol H2 | B��0.2mol Cl2 | C��0.4mol H2 | D��0.4mol Cl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
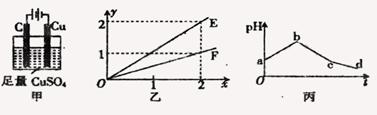
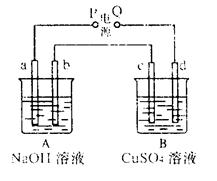
| A��OE��ʾ��Ӧ����CU�����ʵ��� |
| B��OF��ʾ��Ӧ����O2�����ʵ��� |
| C��bc ������������Cl2 |
| D��cd�ε���������ˮ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com