
����Ŀ����PbSǦ����(��Ҫ�ɷ�ΪPbS������������)̼������Ǧ��һ����ɫ���գ�����Ҫ�����������£�

�ش��������⣺
��1����ת�����Ǹù��յĹؼ���ת��ʱ������Ӧ�Ļ�ѧ����ʽΪ2PbS��2(NH4)2CO3��O2��2H2O===2PbCO3��2S��4NH3��H2O��
��(NH4)2CO3��������Ӧ��________(����ĸ)��
a����������b������ԭ��c���Ȳ���������Ҳ������ԭ��
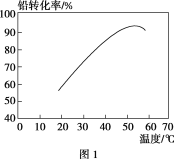
��ת��ʱ���¶ȶ�Ǧת���ʵ�Ӱ����ͼ1��ʾ�������˵�ת���¶ȷ�ΧΪ________��
������FeS2��Ǧת���ʵ�Ӱ����ͼ2��ʾ��˵��FeS2��ת���е�������________��

��2�����ܽ⡱ʱ������Ӧ�����ӷ���ʽΪ________________________________��
��3������⡱ʱ�������ĵ缫��ӦʽΪ___________________________________��
���𰸡�c 50��60 �� ������ PbCO3��2H��===Pb2����CO2����H2O Pb2����2e��===Pb
��������
��1�����������õ����ӣ����ϼ۽��ͣ���ԭ��ʧȥ���ӣ����ϼ����ߣ�
�����˵�ת���¶ȷ�Χ���Ƿ����ĸ���Χ��Ǧת������ߣ�
���ڻ�ѧ��Ӧ���ܸı䷴Ӧ�ﻯѧ��Ӧ���ʣ���ͣ������ı仯ѧƽ�⣬�ұ����������ͻ�ѧ�����ڻ�ѧ��Ӧǰ��û�з����ı�����ʽд�����
��2���ܽ������̼��Ǧ���ᷴӦ����Ǧ�Ρ�������̼��ˮ��
��3�������õ����ӣ����ϼ۽��ͣ�������ԭ��Ӧ��
��1�����������õ����ӣ����ϼ۽��ͣ���ԭ��ʧȥ���ӣ����ϼ����ߣ�(NH4)2CO3�ڷ�Ӧ��Ԫ�صĻ��ϼ�û�䣬��ѡc��
�����˵�ת���¶ȷ�Χ���Ƿ����ĸ���Χ��Ǧת������ߣ����˵�ת���¶ȷ�ΧΪ50��60 ����
���ڻ�ѧ��Ӧ���ܸı䷴Ӧ�ﻯѧ��Ӧ���ʣ���ͣ������ı仯ѧƽ�⣬�ұ����������ͻ�ѧ�����ڻ�ѧ��Ӧǰ��û�з����ı�����ʽд�����FeS2��ת���е���������������
��2���ܽ������̼��Ǧ���ᷴӦ����Ǧ�Ρ�������̼��ˮ�����ܽ⡱ʱ������Ӧ�����ӷ���ʽΪPbCO3��2H��===Pb2����CO2����H2O ��
��3�������õ����ӣ����ϼ۽��ͣ�������ԭ��Ӧ������⡱ʱ�������ĵ缫��ӦʽΪ�� Pb2����2e��===Pb��


 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и�����������ɫ����������Һ���ܴ����������
A. Mg 2+��NO3��C1��K+ B. Na+��Cu2+��Cl��SO42
C. Ca2+��Na+��NO3��MnO4 D. K+ ��Cl ��Na+ ��HCO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����2CH3CHO��O2![]() 2CH3COOH������AΪ��Ҫԭ�Ϻϳɻ�����E����ϳ�·����ͼ1��ʾ���ش��������⣺
2CH3COOH������AΪ��Ҫԭ�Ϻϳɻ�����E����ϳ�·����ͼ1��ʾ���ش��������⣺

ͼ1
(1)д���������ʵĹ��������ƣ�
B��____________________��D��____________________��
(2)��Ӧ�ܵĻ�ѧ����ʽΪ________________________________________________����Ӧ���ͣ�________��
(3)ijѧϰС���������B��������ʵ��װ�����£�����ͼ2װ�ûش����⡣

�� �� �� ��
ͼ2
��װ�ü���ƿ��ʢ�ŵĹ���ҩƷ����Ϊ________(����ĸ)��
A Na2O2 B KCl C Na2CO3 D MnO2
��ʵ������У���װ��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽΪ_________________________��
������B�Ĵ����������������Ǿ�����ͬ��������Ӧ�������õ���������μӵ�����������ͭ����Һ�м��ȣ�����Ϊ______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)������Ԫ��W��X��Y��Z��Mԭ��������������Ԫ��W��һ�ֺ��ص�������Ϊ0��X��ԭ�������������Ǵ�����2����Z��Mͬ���壬Z2�����Ӳ�ṹ����ԭ����ͬ��
��M��Ԫ�����ڱ��е�λ����__________________________��
�ڻ�����p��W��X��Y��M����Ԫ����ɡ���֪��p��Һ�м���FeCl3��Һ����Һ��Ѫ��ɫ����p��Һ�м���NaOH��Һ�����ȿɷų�ʹʪ��ĺ�ɫʯ����ֽ���������塣p�Ļ�ѧʽΪ_____________��
����XY)2��������Cl2���ƣ�(XY)2��NaOH��Һ�����·�Ӧ�����ӷ���ʽΪ_____________��
(2)A��B��C��W��Ϊ��ѧ�����Ĵ��������֮��������ת����ϵ���������P��Ӧ��������ȥ����Ӧ������ˮ��Һ�н��У���
![]()
��. ��AΪ�д̼�����ζ�����壬ˮ��Һ�ʼ��ԣ�CΪ����ɫ���壬��ˮ��Ӧ����һԪǿ��D��D����ǿ�����ԡ�
���ڴ��������£�A��C��Ӧ��������������Ⱦ���ʣ��÷�Ӧ��ѧ����ʽΪ________________
�ڹ�ҵ�ϳ���Na2CO3��Һ����C�����ɵ����ʵ������������Σ��÷�Ӧ�����ӷ���ʽΪ_______________
��. A��B��C�������ʵ���Һ���Լ��ԣ���ɫ��Ӧ��Ϊ��ɫ��C����������ķ��ݼ������ȷֽ������B��
��ʵ����������0.1mol��L��1 A��Һ450mL�����ݼ�����������ƽ��ȡA������Ϊ__________ g����ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��________________0.1mol��L��1 (����������������������С����)��
�ڽ���״����2.24L ��Wͨ��150mL 1mol��L��1��A��Һ�У���ַ�Ӧ���ܷ�Ӧ�����ӷ���ʽΪ_______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)�������������Т�Ne����H2O����NH3����KOH����Na2O��ֻ���ڹ��ۼ�����____��ֻ�������Ӽ�����____���ȴ��ڹ��ۼ��ִ������Ӽ�����____�������ڻ�ѧ������_____������д��ţ�
(2)���Т�Na2S����NH4Cl���۸ɱ����ܵ�Ƭ�������ʣ�������Ҫ��ش�
�õ���ʽ��ʾ�ٵ��γɹ�����_______���õ���ʽ��ʾ�ܵ��γɹ�����___________�ڵĵ���ʽ��________�۵ĵ���ʽ��__________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������̼ѭ������������ĸ߶����ӣ�����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2��������ȫ������ձ����ӡ���CO2ת��Ϊ�״���CO2(g)��3H2(g)CH3OH(g)��H2O(g)��
��1����һ���º����ܱ������г���1 mol CO2��3 mol H2����������Ӧ�����CO2(g)��CH3OH(g)Ũ����ʱ��仯��ͼ��ʾ��

��0��10 min�ڣ�������ƽ����Ӧ����Ϊ____________����10 min�����¶Ȳ��䣬����ܱ��������ٳ���1 mol CO2(g)��1 mol H2O(g)����ƽ��________(�����������)�ƶ���
������֪��CO2(g)��3H2(g)CH3OH(g)��H2O(g)����H����a kJ��mol��1��
2H2(g)��O2(g)===2H2O(g)����H����b kJ��mol��1��
H2O(g)===H2O(l)����H����c kJ��mol��1��
CH3OH(g)===CH3OH(l)����H����d kJ��mol��1��
���ʾCH3OH(l)ȼ���ȵ��Ȼ�ѧ����ʽΪ______________________________________��
��2����ͼ��25 ��ʱ�Լ״�ȼ�ϵ��(�������ҺΪϡ����)Ϊ��Դ�����600 mLһ��Ũ�ȵ�NaCl��Һ����ص�������ӦʽΪ_________________________________��

�ڵ��һ��ʱ���NaCl��Һ�� pH ��Ϊ12(������ǰ��NaCl��Һ���������)�������������ļ״������ʵ���Ϊ________mol��
��3������2��U�ι��ڵ������Һ������NaCl��Һ��ȫ����⣩��ͨ���״����89.6 mL ��CO2���壬��������Һ��________(��ᡱ������С�)�ԣ���Һ�и�����Ũ���ɴ�С��˳��Ϊ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾװ�ý�������ʵ�飬�ܵó���Ӧʵ����۵���
ѡ�� | �� | �� | �� | ʵ����� |
|
A | ϡ���� | Na2SO3 | Na2SiO3�� | �ǽ����ԣ�S>Si | |
B | Ũ���� | ���� | ��ˮ | Ũ���������ˮ�ԡ������� | |
C | ϡ���� | Na2SO3 | Ba(NO3)2��Һ | SO2������Ա��ξ������ɰ�ɫ���� | |
D | Ũ���� | Na2CO3 | Na2SiO3��Һ | ���ԣ�����>̼��>���� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ�鼰�������Ƴ���Ӧ���۵��ǣ� ��
ʵ�� | ���� | ���� | |
A | �� | ��Һ��ΪѪ��ɫ |
|
B | �� | ���ɰ�ɫ���� | �ǽ����� |
C | ��ʢ�� | ��ˮ��ɫ | ��ԭ�ԣ� |
D | ����ɫ��Һ�е��� | �а�ɫ�������� | ��ɫ��Һ��һ���� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ���У���Ӧ�������Լ����۶���ȷ�����߾��������ϵ����
ѡ�� | ʵ�� | ���� | ���� |
A | ��ϡ�����м����������ۣ���ַ�Ӧ��μ�KSCN��Һ | ���������ɣ���Һ��Ѫ��ɫ | ϡ���ὫFe����ΪFe3�� |
B | ��ͭ�ۼ��뵽1.0 mol��L��1 Fe2(SO4)3��Һ�� | ͭ���ܽ⣬��Һ���� | ��������ͭ���� |
C | ��5 mL 0.005 mol��L��1 FeCl3��Һ��5 mL 0.015 mol��L��1 KSCN��Һ��ϣ��ﵽƽ����ٵμ�4��1 mol��L��1��KCl��Һ | ��Һ��ɫ���� | ����Ӧ��Ũ�ȣ�ƽ�������ƶ� |
D | ��10 mL 0.1 mol��L��1 AgNO3��Һ�еμ�4��0.1 mol��L��1 NaCl��Һ��Ȼ���ٵμ�4��0.1 mol��L��1 Na2S��Һ | ���а�ɫ�������ɣ����к�ɫ�������� | ��ͬ�¶��£�Ag2S���ܶȻ���AgCl��С |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com