
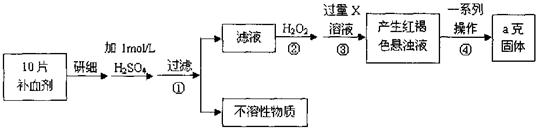
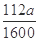 ���仯����ʽ����
���仯����ʽ����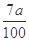 )
)


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
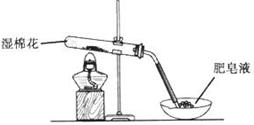
| ���� | ���� | �������� | �������� |
| 1 | ȡ��ɫ��ĩ����ϡ���� | �ܽ⣬������ | �ܽ⣬������ |
| 2 | ȡ����1����Һ���μ�����KMnO4��Һ | ��ɫ��ȥ | ��ɫ��ȥ |
| 3 | ȡ����1����Һ���μ�KSCN��Һ | ��� | ������ |
| 4 | ����3��Һ�еμ�������ˮ | ��ɫ��ȥ | �ȱ�죬����ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
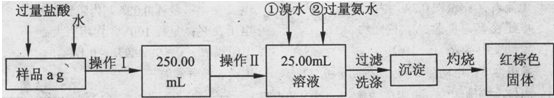
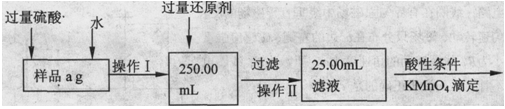
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
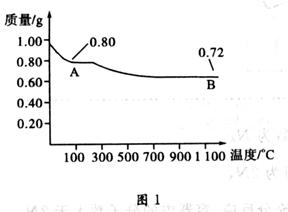

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��11.2g | B��5.6g | C��2.8g | D�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
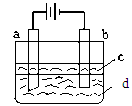
| A����ˮ | B��NaCl��Һ | C��NaOH��Һ | D��CuCl2��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2FeCl2��CuCl2������FeCl3��Һ�м���a g Cu�ۣ���ȫ�ܽ���ټ���b g Fe�ۣ���ַ�Ӧ������c g������塣��c��a��������˵����ȷ����
2FeCl2��CuCl2������FeCl3��Һ�м���a g Cu�ۣ���ȫ�ܽ���ټ���b g Fe�ۣ���ַ�Ӧ������c g������塣��c��a��������˵����ȷ����| A���������ȫ����Cu |
| B������������ΪFe��Cu�Ļ���� |
| C�����õ�����Һ���ܺ���Fe3�� |
| D�����������Fe����Һ��һ������Cu2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
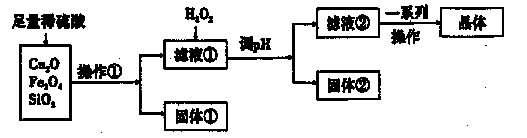
| A��NaOH��Һ | B��CuO | C����ˮ | D��CuCO3 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com